The cyclin-dependent kinase inhibitor Dacapo promotes replication licensing during Drosophila endocycles
- PMID: 17380129
- PMCID: PMC1852775
- DOI: 10.1038/sj.emboj.7601648
The cyclin-dependent kinase inhibitor Dacapo promotes replication licensing during Drosophila endocycles
Abstract
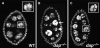
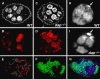
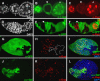
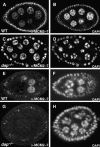
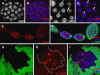
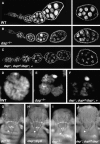
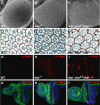
The endocycle is a developmentally programmed variant cell cycle in which cells undergo repeated rounds of DNA replication with no intervening mitosis. In Drosophila, the endocycle is driven by the oscillations of Cyclin E/Cdk2 activity. How the periodicity of Cyclin E/Cdk2 activity is achieved during endocycles is poorly understood. Here, we demonstrate that the p21(cip1)/p27(kip1)/p57(kip2)-like cyclin-dependent kinase inhibitor (CKI), Dacapo (Dap), promotes replication licensing during Drosophila endocycles by reinforcing low Cdk activity during the endocycle Gap-phase. In dap mutants, cells in the endocycle have reduced levels of the licensing factor Double Parked/Cdt1 (Dup/Cdt1), as well as decreased levels of chromatin-bound minichromosome maintenance (MCM2-7) complex. Moreover, mutations in dup/cdt1 dominantly enhance the dap phenotype in several polyploid cell types. Consistent with a reduced ability to complete genomic replication, dap mutants accumulate increased levels of DNA damage during the endocycle S-phase. Finally, genetic interaction studies suggest that dap functions to promote replication licensing in a subset of Drosophila mitotic cycles.
Figures
Similar articles
-
The Cyclin-dependent kinase inhibitor Dacapo promotes genomic stability during premeiotic S phase.Mol Biol Cell. 2009 Apr;20(7):1960-9. doi: 10.1091/mbc.e08-09-0916. Epub 2009 Feb 11. Mol Biol Cell. 2009. PMID: 19211840 Free PMC article.
-
Proper CycE-Cdk2 activity in endocycling tissues requires regulation of the cyclin-dependent kinase inhibitor Dacapo by dE2F1b in Drosophila.Genetics. 2021 Mar 3;217(1):1-15. doi: 10.1093/genetics/iyaa029. Genetics. 2021. PMID: 33683365 Free PMC article.
-
Drosophila double-parked is sufficient to induce re-replication during development and is regulated by cyclin E/CDK2.Development. 2004 Oct;131(19):4807-18. doi: 10.1242/dev.01348. Epub 2004 Sep 1. Development. 2004. PMID: 15342466
-
Endoreplication.Cold Spring Harb Perspect Biol. 2013 Jan 1;5(1):a012948. doi: 10.1101/cshperspect.a012948. Cold Spring Harb Perspect Biol. 2013. PMID: 23284048 Free PMC article. Review.
-
New insights into cell cycle control from the Drosophila endocycle.Oncogene. 2005 Apr 18;24(17):2765-75. doi: 10.1038/sj.onc.1208610. Oncogene. 2005. PMID: 15838513 Review.
Cited by
-
Replication fork progression during re-replication requires the DNA damage checkpoint and double-strand break repair.Curr Biol. 2015 Jun 15;25(12):1654-60. doi: 10.1016/j.cub.2015.04.058. Epub 2015 Jun 4. Curr Biol. 2015. PMID: 26051888 Free PMC article.
-
The GATOR complex regulates an essential response to meiotic double-stranded breaks in Drosophila.Elife. 2019 Oct 25;8:e42149. doi: 10.7554/eLife.42149. Elife. 2019. PMID: 31650955 Free PMC article.
-
CAF-1 is required for efficient replication of euchromatic DNA in Drosophila larval endocycling cells.Chromosoma. 2009 Apr;118(2):235-48. doi: 10.1007/s00412-008-0192-2. Epub 2008 Dec 9. Chromosoma. 2009. PMID: 19066929
-
The CDK regulators Cdh1 and Sic1 promote efficient usage of DNA replication origins to prevent chromosomal instability at a chromosome arm.Nucleic Acids Res. 2014 Jun;42(11):7057-68. doi: 10.1093/nar/gku313. Epub 2014 Apr 21. Nucleic Acids Res. 2014. PMID: 24753426 Free PMC article.
-
Characterization of a Drosophila ortholog of the Cdc7 kinase: a role for Cdc7 in endoreplication independent of Chiffon.J Biol Chem. 2015 Jan 16;290(3):1332-47. doi: 10.1074/jbc.M114.597948. Epub 2014 Dec 1. J Biol Chem. 2015. PMID: 25451925 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

