Structure and enzymatic functions of human CD38
- PMID: 17380198
- PMCID: PMC1829193
- DOI: 10.2119/2006–00086.Lee
Structure and enzymatic functions of human CD38
Abstract
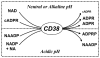
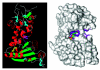
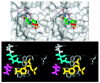
CD38 is a novel multifunctional protein that serves not only as an antigen but also as an enzyme. It catalyzes the metabolism of cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate, two structurally and functionally distinct Ca(2+) messengers targeting, respectively, the endoplasmic reticulum and lysosomal Ca(2+) stores. The protein has recently been crystallized and its three-dimensional structure solved to a resolution of 1.9 A. The crystal structure of a binary complex reveals critical interactions between residues at the active site and a bound substrate, providing mechanistic insights to its novel multi-functional catalysis. This article reviews the current advances in the understanding of the structural determinants that control the multiple enzymatic reactions catalyzed by CD38.
Figures

Similar articles
-
Resolving the topological enigma in Ca2+ signaling by cyclic ADP-ribose and NAADP.J Biol Chem. 2019 Dec 27;294(52):19831-19843. doi: 10.1074/jbc.REV119.009635. Epub 2019 Oct 31. J Biol Chem. 2019. PMID: 31672920 Free PMC article. Review.
-
Identification of the enzymatic active site of CD38 by site-directed mutagenesis.J Biol Chem. 2000 Jul 14;275(28):21566-71. doi: 10.1074/jbc.M909365199. J Biol Chem. 2000. PMID: 10781610
-
Structural basis for formation and hydrolysis of the calcium messenger cyclic ADP-ribose by human CD38.J Biol Chem. 2007 Feb 23;282(8):5853-61. doi: 10.1074/jbc.M609093200. Epub 2006 Dec 20. J Biol Chem. 2007. PMID: 17182614
-
Cyclic ADP-ribose and NAADP: fraternal twin messengers for calcium signaling.Sci China Life Sci. 2011 Aug;54(8):699-711. doi: 10.1007/s11427-011-4197-3. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786193 Review.
-
Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities.J Biol Chem. 2006 Sep 29;281(39):28951-7. doi: 10.1074/jbc.M604370200. Epub 2006 Jul 21. J Biol Chem. 2006. PMID: 16861223
Cited by
-
Paracrine ADP Ribosyl Cyclase-Mediated Regulation of Biological Processes.Cells. 2022 Aug 24;11(17):2637. doi: 10.3390/cells11172637. Cells. 2022. PMID: 36078044 Free PMC article. Review.
-
Monoclonal Antibody: A New Treatment Strategy against Multiple Myeloma.Antibodies (Basel). 2017 Nov 14;6(4):18. doi: 10.3390/antib6040018. Antibodies (Basel). 2017. PMID: 31548533 Free PMC article. Review.
-
Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions.Front Immunol. 2020 Apr 24;11:501. doi: 10.3389/fimmu.2020.00501. eCollection 2020. Front Immunol. 2020. PMID: 32391000 Free PMC article. Review.
-
Daratumumab and its potential in the treatment of multiple myeloma: overview of the preclinical and clinical development.Ther Adv Hematol. 2015 Jun;6(3):120-7. doi: 10.1177/2040620715572295. Ther Adv Hematol. 2015. PMID: 26137203 Free PMC article. Review.
-
Management of cardiovascular risk in patients with multiple myeloma.Blood Cancer J. 2019 Feb 26;9(3):26. doi: 10.1038/s41408-019-0183-y. Blood Cancer J. 2019. PMID: 30808934 Free PMC article. Review.
References
-
- Malavasi F, et al. CD38: a multi-lineage cell activation molecule with a split personality. Int J Clin Lab Res. 1992;22:73–80. - PubMed
-
- Malavasi F, et al. Human CD38: a glycoprotein in search of a function. Immunol Today. 1994;15:95–97. - PubMed
-
- Mehta K, Shahid U, Malavasi F. Human CD38, a cell-surface protein with multiple functions. FASEB J. 1996;10:1408–17. - PubMed
-
- Takasawa S, et al. Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J Biol Chem. 1993;268:26052–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
