Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model
- PMID: 17380206
- PMCID: PMC1821068
- DOI: 10.1172/JCI30537
Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model
Abstract
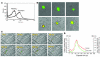
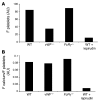
Adhesion of platelets to an injured vessel wall and platelet activation are critical events in the formation of a thrombus. Of the agonists involved in platelet activation, thrombin, collagen, and vWF are known to induce in vitro calcium mobilization in platelets. Using a calcium-sensitive fluorochrome and digital multichannel intravital microscopy to image unstimulated and stimulated platelets, calcium mobilization was monitored as a reporter of platelet activation (as distinct from platelet accumulation) during thrombus formation in live mice. In the absence of vWF, platelet activation was normal, but platelet adherence and aggregation were attenuated during thrombus formation following laser-induced injury in the cremaster muscle microcirculation. In WT mice treated with lepirudin, platelet activation was blocked, and platelet adherence and aggregation were inhibited. The kinetics of platelet activation and platelet accumulation were similar in FcRgamma(-/-) mice lacking glycoprotein VI (GPVI), GPVI-depleted mice, and WT mice. Our results indicate that the tissue factor-mediated pathway of thrombin generation, but not the collagen-induced GPVI-mediated pathway, is the major pathway leading to platelet activation after laser-induced injury under the conditions employed. In the tissue factor-mediated pathway, vWF plays a role in platelet accumulation during thrombus formation but is not required for platelet activation in vivo.
Figures





References
-
- Ruggeri Z.M. Platelets in atherothrombosis. Nat. Med. 2002;8:1227–1234. - PubMed
-
- Gibbins J.M. Platelet adhesion signalling and the regulation of thrombus formation. J. Cell Sci. 2004;117:3415–3425. - PubMed
-
- Jackson S.P., Nesbitt W.S., Kulkarni S. Signaling events underlying thrombus formation. J. Thromb. Haemost. 2003;1:1602–1612. - PubMed
-
- Savage B., Saldivar E., Ruggeri Z.M. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

