Bystin in human cancer cells: intracellular localization and function in ribosome biogenesis
- PMID: 17381424
- PMCID: PMC1896285
- DOI: 10.1042/BJ20061597
Bystin in human cancer cells: intracellular localization and function in ribosome biogenesis
Abstract
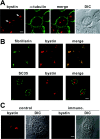
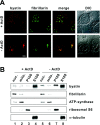
Although bystin has been identified as a protein potentially involved in embryo implantation (a process unique to mammals) in humans, the bystin gene is evolutionarily conserved from yeast to humans. DNA microarray data indicates that bystin is overexpressed in human cancers, suggesting that it promotes cell growth. We undertook RT (reverse transcription)-PCR and immunoblotting, and confirmed that bystin mRNA and protein respectively are expressed in human cancer cell lines, including HeLa. Subcellular fractionation identified bystin protein as nuclear and cytoplasmic, and immunofluorescence showed that nuclear bystin localizes mainly in the nucleolus. Sucrose gradient ultracentrifugation of total cytoplasmic ribosomes revealed preferential association of bystin with the 40S subunit fractions. To analyse its function, bystin expression in cells was suppressed by RNAi (RNA interference). Pulse-chase analysis of ribosomal RNA processing suggested that bystin knockdown delays processing of 18S ribosomal RNA, a component of the 40S subunit. Furthermore, this knockdown significantly inhibited cell proliferation. Our findings suggest that bystin may promote cell proliferation by facilitating ribosome biogenesis, specifically in the production of the 40S subunit. Localization of bystin to the nucleolus, the site of ribosome biogenesis, was blocked by low concentrations of actinomycin D, a reagent that causes nucleolar stress. When bystin was transiently overexpressed in HeLa cells subjected to nucleolar stress, nuclear bystin was included in particles different from the nuclear stress granules induced by heat shock. In contrast, cytoplasmic bystin was barely affected by nucleolar stress. These results suggest that, while bystin may play multiple roles in mammalian cells, a conserved function is to facilitate ribosome biogenesis required for cell growth.
Figures






Similar articles
-
Bystin-like protein is upregulated in hepatocellular carcinoma and required for nucleologenesis in cancer cell proliferation.Cell Res. 2009 Oct;19(10):1150-64. doi: 10.1038/cr.2009.99. Epub 2009 Aug 18. Cell Res. 2009. PMID: 19687802
-
Implication of nucleolar protein SURF6 in ribosome biogenesis and preimplantation mouse development.Biol Reprod. 2006 Nov;75(5):690-6. doi: 10.1095/biolreprod.106.054072. Epub 2006 Jul 19. Biol Reprod. 2006. PMID: 16855206
-
The role of bystin in embryo implantation and in ribosomal biogenesis.Cell Mol Life Sci. 2008 Jan;65(1):92-9. doi: 10.1007/s00018-007-7302-9. Cell Mol Life Sci. 2008. PMID: 17917702 Free PMC article. Review.
-
Roles of eukaryotic ribosomal proteins in maturation and transport of pre-18S rRNA and ribosome function.Mol Cell. 2005 Oct 28;20(2):263-75. doi: 10.1016/j.molcel.2005.09.005. Mol Cell. 2005. PMID: 16246728
-
Nuclear export and cytoplasmic maturation of ribosomal subunits.FEBS Lett. 2007 Jun 19;581(15):2783-93. doi: 10.1016/j.febslet.2007.05.013. Epub 2007 May 11. FEBS Lett. 2007. PMID: 17509569 Review.
Cited by
-
Trophinin-mediated cell adhesion induces apoptosis of human endometrial epithelial cells through PKC-δ.Cell Cycle. 2011 Jan 1;10(1):135-43. doi: 10.4161/cc.10.1.14448. Epub 2011 Jan 1. Cell Cycle. 2011. PMID: 21191175 Free PMC article.
-
Lyar, a cell growth-regulating zinc finger protein, was identified to be associated with cytoplasmic ribosomes in male germ and cancer cells.Mol Cell Biochem. 2014 Oct;395(1-2):221-9. doi: 10.1007/s11010-014-2128-x. Epub 2014 Jul 3. Mol Cell Biochem. 2014. PMID: 24990247
-
Polycystic Ovary Syndrome and Ferroptosis: Following Ariadne's Thread.Biomedicines. 2024 Oct 8;12(10):2280. doi: 10.3390/biomedicines12102280. Biomedicines. 2024. PMID: 39457593 Free PMC article.
-
The human WBSCR22 protein is involved in the biogenesis of the 40S ribosomal subunits in mammalian cells.PLoS One. 2013 Sep 23;8(9):e75686. doi: 10.1371/journal.pone.0075686. eCollection 2013. PLoS One. 2013. PMID: 24086612 Free PMC article.
-
Ribosomal protein S15A augments human osteosarcoma cell proliferation in vitro.Cancer Biother Radiopharm. 2014 Dec;29(10):451-6. doi: 10.1089/cbr.2014.1698. Cancer Biother Radiopharm. 2014. Retraction in: Cancer Biother Radiopharm. 2021 Dec;36(10):889. doi: 10.1089/cbr.2014.1698.retract. PMID: 25409460 Free PMC article. Retracted.
References
-
- Carson D. D., Bagchi I., Dey S. K., Enders A. C., Fazleabas A. T., Lessey B. A., Yoshinaga K. Embryo implantation. Dev. Biol. 2000;223:217–237. - PubMed
-
- Cross J. C., Werb Z., Fisher S. J. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. - PubMed
-
- Strickland S., Richards W. G. Invasion of the trophoblasts. Cell. 1992;71:355–357. - PubMed
-
- Murray M. J., Lessey B. A. Embryo implantation and tumor metastasis: common pathways of invasion and angiogenesis. Semin. Reprod. Endocrinol. 1999;17:275–290. - PubMed
-
- Fukuda M. N., Sato T., Nakayama J., Klier G., Mikami M., Aoki D., Nozawa S. Trophinin and tastin, a novel cell adhesion molecule complex with potential involvement in embryo implantation. Genes Dev. 1995;9:1199–1210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

