Substrate specificity and catalytic efficiency of aldo-keto reductases with phospholipid aldehydes
- PMID: 17381426
- PMCID: PMC1925154
- DOI: 10.1042/BJ20061743
Substrate specificity and catalytic efficiency of aldo-keto reductases with phospholipid aldehydes
Abstract
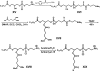
Phospholipid oxidation generates several bioactive aldehydes that remain esterified to the glycerol backbone ('core' aldehydes). These aldehydes induce endothelial cells to produce monocyte chemotactic factors and enhance monocyte-endothelium adhesion. They also serve as ligands of scavenger receptors for the uptake of oxidized lipoproteins or apoptotic cells. The biochemical pathways involved in phospholipid aldehyde metabolism, however, remain largely unknown. In the present study, we have examined the efficacy of the three mammalian AKR (aldo-keto reductase) families in catalysing the reduction of phospholipid aldehydes. The model phospholipid aldehyde POVPC [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine] was efficiently reduced by members of the AKR1, but not by the AKR6 or the ARK7 family. In the AKR1 family, POVPC reductase activity was limited to AKR1A and B. No significant activity was observed with AKR1C enzymes. Among the active proteins, human AR (aldose reductase) (AKR1B1) showed the highest catalytic activity. The catalytic efficiency of human small intestinal AR (AKR1B10) was comparable with the murine AKR1B proteins 1B3 and 1B8. Among the murine proteins AKR1A4 and AKR1B7 showed appreciably lower catalytic activity as compared with 1B3 and 1B8. The human AKRs, 1B1 and 1B10, and the murine proteins, 1B3 and 1B8, also reduced C-7 and C-9 sn-2 aldehydes as well as POVPE [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphoethanolamine]. AKR1A4, B1, B7 and B8 catalysed the reduction of aldehydes generated in oxidized C(16:0-20:4) phosphatidylcholine with acyl, plasmenyl or alkyl linkage at the sn-1 position or C(16:0-20:4) phosphatidylglycerol or phosphatidic acid. AKR1B1 displayed the highest activity with phosphatidic acids; AKR1A4 was more efficient with long-chain aldehydes such as 5-hydroxy-8-oxo-6-octenoyl derivatives, whereas AKR1B8 preferred phosphatidylglycerol. These results suggest that proteins of the AKR1A and B families are efficient phospholipid aldehyde reductases, with non-overlapping substrate specificity, and may be involved in tissue-specific metabolism of endogenous or dietary phospholipid aldehydes.
Figures




Similar articles
-
Aldose reductase-catalyzed reduction of aldehyde phospholipids.J Biol Chem. 2004 Dec 17;279(51):53395-406. doi: 10.1074/jbc.M403416200. Epub 2004 Oct 1. J Biol Chem. 2004. PMID: 15465833 Free PMC article.
-
Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members.Biochem J. 1999 Oct 15;343 Pt 2(Pt 2):487-504. Biochem J. 1999. PMID: 10510318 Free PMC article.
-
Involvement of aldose reductase in the metabolism of atherogenic aldehydes.Chem Biol Interact. 2001 Jan 30;130-132(1-3):563-71. doi: 10.1016/s0009-2797(00)00299-4. Chem Biol Interact. 2001. PMID: 11306075
-
The aldo-keto reductases (AKRs): Overview.Chem Biol Interact. 2015 Jun 5;234:236-46. doi: 10.1016/j.cbi.2014.09.024. Epub 2014 Oct 7. Chem Biol Interact. 2015. PMID: 25304492 Free PMC article. Review.
-
Gene regulation of aldose-, aldehyde- and a renal specific oxido reductase (RSOR) in the pathobiology of diabetes mellitus.Curr Med Chem. 2003 Aug;10(15):1399-406. doi: 10.2174/0929867033457368. Curr Med Chem. 2003. PMID: 12871137 Review.
Cited by
-
Reductive metabolism of AGE precursors: a metabolic route for preventing AGE accumulation in cardiovascular tissue.Diabetes. 2009 Nov;58(11):2486-97. doi: 10.2337/db09-0375. Epub 2009 Aug 3. Diabetes. 2009. PMID: 19651811 Free PMC article.
-
Aldo-Keto Reductase Family 1 Member B10 Inhibitors: Potential Drugs for Cancer Treatment.Recent Pat Anticancer Drug Discov. 2016;11(2):184-96. doi: 10.2174/1574892811888160304113346. Recent Pat Anticancer Drug Discov. 2016. PMID: 26844556 Free PMC article. Review.
-
Tissue distribution, ontogeny, and chemical induction of aldo-keto reductases in mice.Drug Metab Dispos. 2013 Aug;41(8):1480-7. doi: 10.1124/dmd.113.051904. Epub 2013 May 9. Drug Metab Dispos. 2013. PMID: 23660342 Free PMC article.
-
Catalytic mechanism and substrate specificity of the beta-subunit of the voltage-gated potassium channel.Biochemistry. 2008 Aug 26;47(34):8840-54. doi: 10.1021/bi800301b. Epub 2008 Aug 2. Biochemistry. 2008. PMID: 18672894 Free PMC article.
-
AKR1B10 dictates c-Myc stability to suppress colorectal cancer metastasis via PP2A nitration.Sci Adv. 2025 Aug 22;11(34):eadv6937. doi: 10.1126/sciadv.adv6937. Epub 2025 Aug 22. Sci Adv. 2025. PMID: 40845116 Free PMC article.
References
-
- Porter N. A., Caldwell S. E., Mills K. A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. - PubMed
-
- Glass C. K., Witztum J. L. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. - PubMed
-
- Montine T. J., Neely M. D., Quinn J. F., Beal M. F., Markesbery W. R., Roberts L. J., Morrow J. D. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radical Biol. Med. 2002;33:620–626. - PubMed
-
- Shinmura K., Bolli R., Liu S. Q., Tang X. L., Kodani E., Xuan Y. T., Srivastava S., Bhatnagar A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ. Res. 2002;91:240–246. - PubMed
-
- Srivastava S., Chandrasekar B., Bhatnagar A., Prabhu S. D. Lipid peroxidation-derived aldehydes and oxidative stress in the failing heart: role of aldose reductase. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2612–H2619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

