Loss of stem cell regenerative capacity within aged niches
- PMID: 17381551
- PMCID: PMC2063610
- DOI: 10.1111/j.1474-9726.2007.00286.x
Loss of stem cell regenerative capacity within aged niches
Abstract
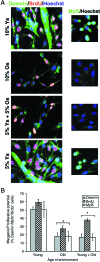
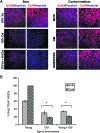
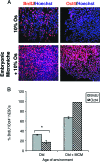
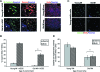
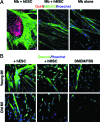
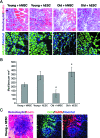
This work uncovers novel mechanisms of aging within stem cell niches that are evolutionarily conserved between mice and humans and affect both embryonic and adult stem cells. Specifically, we have examined the effects of aged muscle and systemic niches on key molecular identifiers of regenerative potential of human embryonic stem cells (hESCs) and post-natal muscle stem cells (satellite cells). Our results reveal that aged differentiated niches dominantly inhibit the expression of Oct4 in hESCs and Myf-5 in activated satellite cells, and reduce proliferation and myogenic differentiation of both embryonic and tissue-specific adult stem cells (ASCs). Therefore, despite their general neoorganogenesis potential, the ability of hESCs, and the more differentiated myogenic ASCs to contribute to tissue repair in the old will be greatly restricted due to the conserved inhibitory influence of aged differentiated niches. Significantly, this work establishes that hESC-derived factors enhance the regenerative potential of both young and, importantly, aged muscle stem cells in vitro and in vivo; thus, suggesting that the regenerative outcome of stem cell-based replacement therapies will be determined by a balance between negative influences of aged tissues on transplanted cells and positive effects of embryonic cells on the endogenous regenerative capacity. Comprehensively, this work points toward novel venues for in situ restoration of tissue repair in the old and identifies critical determinants of successful cell-replacement therapies for aged degenerating organs.
Figures
References
-
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am. J. Physiol. 1989;256:C1262–C1266. - PubMed
-
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. - PubMed
-
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell. 2002;3:397–409. - PubMed
-
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

