Gcm2 is required for the differentiation and survival of parathyroid precursor cells in the parathyroid/thymus primordia
- PMID: 17382312
- PMCID: PMC1931567
- DOI: 10.1016/j.ydbio.2007.02.014
Gcm2 is required for the differentiation and survival of parathyroid precursor cells in the parathyroid/thymus primordia
Abstract
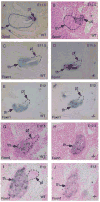
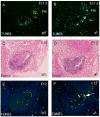
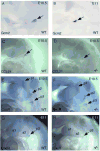
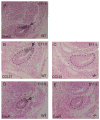
The parathyroid glands develop with the thymus from bilateral common primordia that develop from the 3rd pharyngeal pouch endoderm in mouse embryos at about E11, each of which separates into one parathyroid gland and one thymus lobe by E13.5. Gcm2, a mouse ortholog of the Drosophila Glial Cells Missing gene, is expressed in the parathyroid-specific domains in the 3rd pouches from E9.5. The null mutation of Gcm2 causes aparathyroidism in the fetal and adult mouse and has been proposed to be a master regulator for parathyroid development. In order to study how Gcm2 functions in parathyroid development, we investigated the mechanism that causes the loss of parathyroids in Gcm2 null mutants. Analysis of the 3rd pouch-derived primordium in Gcm2-/- mutants showed the parathyroid-specific domain was present before E12.5 but underwent programmed cell death between E12 and 12.5. RNA and protein localization studies for parathyroid hormone (Pth) in wild-type embryos showed that the presumptive parathyroid domain in the parathyroid/thymus primordia started to transcribe Pth mRNA and produce PTH protein from E11.5 before the separation of parathyroid and thymus domains. However in Gcm2-/- mutants, the parathyroid-specific domain in the common primordium did not express Pth and could not maintain the expression of two other parathyroid marker genes, CasR and CCL21, although expression of these two genes was initiated. Marker gene analysis placed Gcm2 downstream of the known transcription and signaling pathways for parathyroid/thymus organogenesis. These results suggest that Gcm2 is not required for pouch patterning or to establish the parathyroid domain, but is required for differentiation and subsequent survival of parathyroid cells.
Figures
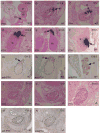
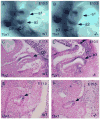

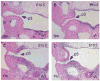
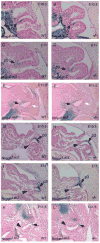
References
-
- Ahn TG, Antonarakis SE, Kronenberg HM, Igarashi T, Levine MA. Familial isolated hypoparathyroidism: a molecular genetic analysis of 8 families with 23 affected persons. Medicine (Baltimore) 1986;65:73–81. - PubMed
-
- Balling R, Erben RG. From parathyroid to thymus, via glial cells. Nat Med. 2000;6:860–1. - PubMed
-
- Berg JP. A molecular switch for parathyroid cell differentiation. Eur J Endocrinol. 2002;146:281–2. - PubMed
-
- Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4:278–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

