Augmented erythrocyte band-3 phosphorylation in septic mice
- PMID: 17382523
- PMCID: PMC1892314
- DOI: 10.1016/j.bbadis.2007.02.004
Augmented erythrocyte band-3 phosphorylation in septic mice
Abstract
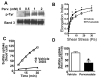
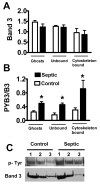
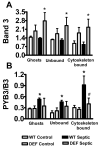
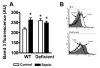
Infection-induced RBC dysfunction has been shown to play a role in the modulation of host response to injury and infection. The underlying biochemical mechanisms are not known. This study investigated alterations in RBC band-3 phosphorylation status and its relationship to anion exchange activity in vitro as well as under in vivo septic conditions induced by cecal ligation and puncture (CLP) in mice. Pervanadate treatment in vitro increased band-3 tyrosine phosphorylation that was accompanied by decreased RBC deformability and anion exchange activity. Following sepsis, band-3 tyrosine phosphorylation in whole RBC ghosts as well as in cytoskeleton-bound or soluble RBC protein fractions were elevated as compared to controls. Although anion exchange activity was similar in RBCs from septic and control animals, band-3 interaction with eosin-5-maleimide (EMA), which binds to band-3 lysine moieties, was increased in cells from septic animals as compared to controls, indicating that sepsis altered band 3 organization within the RBC membrane. Since glucose-6-phosphate dehydrogenase is a major antioxidant enzyme in RBC, in order to assess the potential role of oxidative stress in band-3 tyrosine phosphorylation, sepsis-induced RBC responses were also compared between WT and (G6PD) mutant animals (20% of normal G6PD activity). Band-3 membrane content and EMA staining were elevated in G6PD mutant mice compared to WT under control non-septic conditions. Following sepsis, G6PD mutant animals showed lessened responses in band-3 tyrosine phosphorylation and EMA staining compared to WT. RBC anion exchange activity was similar between mutant and WT animals under all tested conditions. In summary, these studies indicate that sepsis results in elevated band-3 tyrosine phosphorylation and alters band-3 membrane organization without grossly affecting RBC anion exchange activity. The observations also suggest that factors other than oxidative stress are responsible for the sepsis-induced increase in RBC band-3 tyrosine phosphorylation.
Figures

Similar articles
-
Red blood cell dysfunction in septic glucose-6-phosphate dehydrogenase-deficient mice.Am J Physiol Heart Circ Physiol. 2004 Jun;286(6):H2118-26. doi: 10.1152/ajpheart.01085.2003. Epub 2004 Jan 29. Am J Physiol Heart Circ Physiol. 2004. PMID: 14751857
-
Band 3 tyr-phosphorylation in normal and glucose-6-phospate dehydrogenase-deficient human erythrocytes.Mol Membr Biol. 2005 Sep-Oct;22(5):411-20. doi: 10.1080/09687860500233679. Mol Membr Biol. 2005. PMID: 16308275
-
Modifications in erythrocyte membrane protein content are not responsible for the alterations in rheology seen in sepsis.Shock. 2012 Jan;37(1):17-21. doi: 10.1097/SHK.0b013e318237d55a. Shock. 2012. PMID: 21941224 Clinical Trial.
-
Band 3/complement-mediated recognition and removal of normally senescent and pathological human erythrocytes.Cell Physiol Biochem. 2005;16(4-6):133-46. doi: 10.1159/000089839. Cell Physiol Biochem. 2005. PMID: 16301814 Review.
-
The Effect of Sepsis on the Erythrocyte.Int J Mol Sci. 2017 Sep 8;18(9):1932. doi: 10.3390/ijms18091932. Int J Mol Sci. 2017. PMID: 28885563 Free PMC article. Review.
Cited by
-
Red Blood Cell Dysfunction in Critical Illness.Crit Care Clin. 2020 Apr;36(2):267-292. doi: 10.1016/j.ccc.2019.12.008. Epub 2020 Feb 11. Crit Care Clin. 2020. PMID: 32172813 Free PMC article. Review.
-
Ligation of erythrocyte CR1 induces its clustering in complex with scaffolding protein FAP-1.Blood. 2008 Oct 15;112(8):3465-73. doi: 10.1182/blood-2008-04-151845. Epub 2008 Aug 6. Blood. 2008. PMID: 18684861 Free PMC article.
-
Squeezing for Life - Properties of Red Blood Cell Deformability.Front Physiol. 2018 Jun 1;9:656. doi: 10.3389/fphys.2018.00656. eCollection 2018. Front Physiol. 2018. PMID: 29910743 Free PMC article. Review.
-
Ex Vivo Lung Perfusion Model to Study Pulmonary Tissue Extracellular Microvesicle Profiles.Ann Thorac Surg. 2017 Jun;103(6):1758-1766. doi: 10.1016/j.athoracsur.2016.11.074. Epub 2017 Feb 24. Ann Thorac Surg. 2017. PMID: 28242077 Free PMC article.
-
Red blood cell clearance in inflammation.Transfus Med Hemother. 2012 Oct;39(5):353-61. doi: 10.1159/000342229. Epub 2012 Sep 6. Transfus Med Hemother. 2012. PMID: 23801928 Free PMC article.
References
-
- Langenfeld JE, Machiedo GW, Lyons M, Rush BFJ, Dikdan G, Lysz TW. Correlation between red blood cell deformability and changes in hemodynamic function. Surgery. 1994;116:859–867. - PubMed
-
- Powell RJ, Machiedo GW, Rush BFJ. Decreased red blood cell deformability and impaired oxygen utilization during human sepsis. Am. Surg. 1993;59:65–68. - PubMed
-
- Machiedo GW, Powell RJ, Rush BFJ, Swislocki NI, Dikdan G. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple-system organ failure. Arch. Surg. 1989;124:1386–1389. - PubMed
-
- Bateman RM, Jagger JE, Sharpe MD, Ellsworth ML, Mehta S, Ellis CG. Erythrocyte deformability is a nitric oxide-mediated factor in decreased capillary density during sepsis. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2848–H2856. - PubMed
-
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann. Emerg. Med. 1999;34:646–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

