The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial
- PMID: 17382827
- PMCID: PMC2080688
- DOI: 10.1016/S0140-6736(07)60460-7
The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial
Abstract
Background: Carbamazepine is widely accepted as a drug of first choice for patients with partial onset seizures. Several newer drugs possess efficacy against these seizure types but previous randomised controlled trials have failed to inform a choice between these drugs. We aimed to assess efficacy with regards to longer-term outcomes, quality of life, and health economic outcomes.
Methods: SANAD was an unblinded randomised controlled trial in hospital-based outpatient clinics in the UK. Arm A recruited 1721 patients for whom carbamazepine was deemed to be standard treatment, and they were randomly assigned to receive carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate. Primary outcomes were time to treatment failure, and time to 12-months remission, and assessment was by both intention to treat and per protocol. This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN38354748.
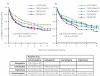
Findings: For time to treatment failure, lamotrigine was significantly better than carbamazepine (hazard ratio [HR] 0.78 [95% CI 0.63-0.97]), gabapentin (0.65 [0.52-0.80]), and topiramate (0.64 [0.52-0.79]), and had a non-significant advantage compared with oxcarbazepine (1.15 [0.86-1.54]). For time to 12-month remission carbamazepine was significantly better than gabapentin (0.75 [0.63-0.90]), and estimates suggest a non-significant advantage for carbamazepine against lamotrigine (0.91 [0.77-1.09]), topiramate (0.86 [0.72-1.03]), and oxcarbazepine (0.92 [0.73-1.18]). In a per-protocol analysis, at 2 and 4 years the difference (95% CI) in the proportion achieving a 12-month remission (lamotrigine-carbamazepine) is 0 (-8 to 7) and 5 (-3 to 12), suggesting non-inferiority of lamotrigine compared with carbamazepine.
Interpretation: Lamotrigine is clinically better than carbamazepine, the standard drug treatment, for time to treatment failure outcomes and is therefore a cost-effective alternative for patients diagnosed with partial onset seizures.
Figures


Comment in
-
First-choice drug for newly diagnosed epilepsy.Lancet. 2007 Mar 24;369(9566):970-1. doi: 10.1016/S0140-6736(07)60470-X. Lancet. 2007. PMID: 17382806 No abstract available.
-
Are the first-line recommendations for antiepileptic drug therapy still valid?Nat Clin Pract Neurol. 2007 Sep;3(9):484-5. doi: 10.1038/ncpneuro0557. Epub 2007 Jul 10. Nat Clin Pract Neurol. 2007. PMID: 17622230 No abstract available.
-
Old versus new antiepileptic drugs: the SANAD study.Lancet. 2007 Jul 28;370(9584):313-4; author reply 315-6. doi: 10.1016/S0140-6736(07)61150-7. Lancet. 2007. PMID: 17662869 No abstract available.
-
Old versus new antiepileptic drugs: the SANAD study.Lancet. 2007 Jul 28;370(9584):313; author reply 315-6. doi: 10.1016/S0140-6736(07)61149-0. Lancet. 2007. PMID: 17662870 No abstract available.
-
Old versus new antiepileptic drugs: the SANAD study.Lancet. 2007 Jul 28;370(9584):314; author reply 315-6. doi: 10.1016/S0140-6736(07)61151-9. Lancet. 2007. PMID: 17662871 No abstract available.
-
Old versus new antiepileptic drugs: the SANAD study.Lancet. 2007 Jul 28;370(9584):314-5; author reply 315-6. doi: 10.1016/S0140-6736(07)61152-0. Lancet. 2007. PMID: 17662872 No abstract available.
-
Lamotrigine was more effective than carbamazepine, gabapentin, and topiramate for treatment failure in partial epilepsy.ACP J Club. 2007 Nov-Dec;147(3):74-5. ACP J Club. 2007. PMID: 17975876 No abstract available.
-
Now we know the drug of first choice--or do we?Epilepsy Curr. 2007 Sep-Oct;7(5):125-7. doi: 10.1111/j.1535-7511.2007.00197.x. Epilepsy Curr. 2007. PMID: 17998970 Free PMC article. No abstract available.
-
Clinical trials in epilepsy: the SANAD study.Rev Neurol Dis. 2008 Winter;5(1):36-7. Rev Neurol Dis. 2008. PMID: 18418324 No abstract available.
References
-
- Hauser WA, Hesdorffer DC. Epilepsy: frequency, causes and consequences. New York: Demos Publications; 1990.
-
- Annegers JF, Hauser WA, Elveback LR. Remission of seizures and relapse in patients with epilepsy. Epilepsia. 1979;20:729–37. - PubMed
-
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet. 1995;346:140–44. - PubMed
-
- London: National Institute for Clinical Excellence; 2004. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. (Clinical Guideline 20).
-
- French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs i: treatment of new onset epilepsy: report of the therapeutics and technology assessment subcommittee and quality standards subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62:1252–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

