Potential anti-inflammatory actions of the elmiric (lipoamino) acids
- PMID: 17383881
- PMCID: PMC1896102
- DOI: 10.1016/j.bmc.2007.03.026
Potential anti-inflammatory actions of the elmiric (lipoamino) acids
Abstract
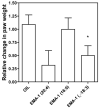
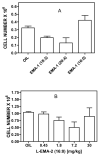
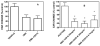
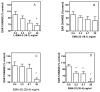
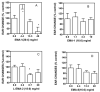
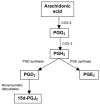
A library of amino acid-fatty acid conjugates (elmiric acids) was synthesized and evaluated for activity as potential anti-inflammatory agents. The compounds were tested in vitro for their effects on cell proliferation and prostaglandin production, and compared with their effects on in vivo models of inflammation. LPS stimulated RAW 267.4 mouse macrophage cells were the in vitro model and phorbol ester-induced mouse ear edema served as the principal in vivo model. The prostaglandin responses were found to be strongly dependent on the nature of the fatty acid part of the molecule. Polyunsaturated acid conjugates produced a marked increase in media levels of i15-deoxy-PGJ(2) with minimal effects on PGE production. It is reported in the literature that prostaglandin ratios in which the J series predominates over the E series promote the resolution of inflammatory conditions. Several of the elmiric acids tested here produced such favorable ratios suggesting that their potential anti-inflammatory activity occurs via a novel mechanism of action. The ear edema assay results were generally in agreement with the prostaglandin assay findings indicating a connection between them.
Figures
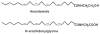
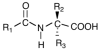
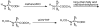
Similar articles
-
Mechanism of anti-inflammatory activity of umbelliferone 6-carboxylic acid isolated from Angelica decursiva.J Ethnopharmacol. 2012 Oct 31;144(1):175-81. doi: 10.1016/j.jep.2012.08.048. Epub 2012 Sep 8. J Ethnopharmacol. 2012. PMID: 22981803
-
Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK inactivation.Eur J Pharmacol. 2008 May 31;586(1-3):340-9. doi: 10.1016/j.ejphar.2008.02.044. Epub 2008 Feb 26. Eur J Pharmacol. 2008. PMID: 18378223
-
Evaluation of anti-inflammatory activity of hydroethanolic extract of Dilodendron bipinnatum Radlk.J Ethnopharmacol. 2014 Aug 8;155(1):387-95. doi: 10.1016/j.jep.2014.05.041. Epub 2014 Jun 12. J Ethnopharmacol. 2014. PMID: 24930355
-
In vitro and in vivo anti-inflammatory effects of taheebo, a water extract from the inner bark of Tabebuia avellanedae.J Ethnopharmacol. 2008 Sep 2;119(1):145-52. doi: 10.1016/j.jep.2008.06.016. Epub 2008 Jun 27. J Ethnopharmacol. 2008. PMID: 18634864
-
Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale.Biochimie. 2009 Jun;91(6):791-5. doi: 10.1016/j.biochi.2009.01.008. Biochimie. 2009. PMID: 19455748 Review.
Cited by
-
Identification of endogenous acyl amino acids based on a targeted lipidomics approach.J Lipid Res. 2010 Jan;51(1):112-9. doi: 10.1194/jlr.M900198-JLR200. J Lipid Res. 2010. PMID: 19584404 Free PMC article.
-
Cannabinoids, endocannabinoids, and related analogs in inflammation.AAPS J. 2009 Mar;11(1):109-19. doi: 10.1208/s12248-009-9084-5. Epub 2009 Feb 6. AAPS J. 2009. PMID: 19199042 Free PMC article. Review.
-
Resolution of inflammation by N-arachidonoylglycine.J Cell Biochem. 2011 Nov;112(11):3227-33. doi: 10.1002/jcb.23245. J Cell Biochem. 2011. PMID: 21732409 Free PMC article.
-
High-performance liquid chromatography-tandem mass spectrometry method for the analysis of N-oleoyl glycine and N-oleoyl alanine in brain and plasma.J Sep Sci. 2023 Nov;46(22):e2300395. doi: 10.1002/jssc.202300395. Epub 2023 Sep 8. J Sep Sci. 2023. PMID: 37688356 Free PMC article.
-
Asymmetric synthesis of novel N-(1-phenyl-2,3-dihydroxypropyl)arachidonylamides and evaluation of their anti-inflammatory activity.Life Sci. 2013 Mar 19;92(8-9):506-11. doi: 10.1016/j.lfs.2012.06.040. Epub 2012 Jul 20. Life Sci. 2013. PMID: 22820546 Free PMC article.
References
-
- Burstein SH, Rossetti RG, Yagen B, Zurier RB. Prostaglandins Other Lipid Mediat. 2000;61:29. - PubMed
-
- Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L, Burstein S, Di Marzo V, Walker JM. J Biol Chem. 2001;276:42639. - PubMed
-
- Burstein SH, Huang SM, Petros TJ, Rossetti RG, Walker JM, Zurier RB. Biochem Pharmacol. 2002;64:1147. - PubMed
-
- Batrakov SG, Mosezhnyi AE, Ruzhitsky AO, Sheichenko VI, Nikitin DI. Biochim Biophys Acta. 2000;1484:225. - PubMed
-
- Kawai Y, Akagawa K, Yano I. Adv Exp Med Biol. 1990;256:159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

