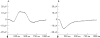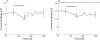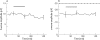The retinal tolerance to bevacizumab in co-application with a recombinant tissue plasminogen activator
- PMID: 17383998
- PMCID: PMC1954819
- DOI: 10.1136/bjo.2006.111260
The retinal tolerance to bevacizumab in co-application with a recombinant tissue plasminogen activator
Abstract
Aim: To investigate the retinal toxicity of bevacizumab in co-application with a commercially available recombinant tissue plasminogen activator (rt-PA), and to facilitate a new therapeutic concept in the treatment of massive subretinal haemorrhage caused by neovascular age-related macular degeneration (AMD).
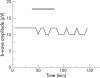
Methods: Isolated bovine retinas were perfused with an oxygen-preincubated nutrient solution. The electroretinogram (ERG) was recorded as a transretinal potential using Ag/AgCl electrodes. Bevacizumab (0.25 mg/ml) and rt-PA (20 microg/ml) were added to the nutrient solution for 45 min. Thereafter, the retina was reperfused for 60 min with normal nutrient solution. Similarly, the effects of rt-PA (20 microg/ml, 60 microg/ml and 200 mug/ml) on the a- and b-wave amplitudes were investigated. The percentages of a- and b-wave reduction during application and at washout were calculated.
Results: During application of bevacizumab (0.25 mg/ml) in co-application with 20 microg/ml (rt-PA), the ERG amplitudes remained stable. The concentrations of rt-PA alone (20 microg/ml and 60 microg/ml) did not induce significant reduction of the b-wave amplitude. In addition, 20 microg/ml rt-PA did not alter the a-wave amplitude. However, 60 microg/ml rt-PA caused a slight but significant reduction of the a-wave amplitude. A full recovery was detected for both concentrations during the washout. At the highest tested concentration of 200 microg/ml rt-PA, a significant reduction of the a- and b-wave amplitudes was provoked during the exposure. The reduction of ERG amplitudes remained irreversible during the washout.
Conclusion: The present study suggests that a subretinal injection of 20 microg/ml rt-PA in co-application with bevacizumab (0.25 mg/ml) for the treatment of massive subretinal haemorrhage seems possible. This is a safety study. Therefore, we did not test the clinical effectiveness of this combined treatment.
Conflict of interest statement
Competing interests: None declared.
Similar articles
-
Effects of bevacizumab on retinal function in isolated vertebrate retina.Br J Ophthalmol. 2006 Sep;90(9):1178-82. doi: 10.1136/bjo.2006.094995. Epub 2006 Jun 5. Br J Ophthalmol. 2006. PMID: 16754646 Free PMC article.
-
The effects of ranibizumab (Lucentis) on retinal function in isolated perfused vertebrate retina.Br J Ophthalmol. 2009 Oct;93(10):1396-400. doi: 10.1136/bjo.2009.157511. Epub 2009 Jul 23. Br J Ophthalmol. 2009. PMID: 19628500
-
The effects of triamcinolone crystals on retinal function in a model of isolated perfused vertebrate retina.Exp Eye Res. 2008 Jul;87(1):22-9. doi: 10.1016/j.exer.2008.04.005. Epub 2008 Apr 23. Exp Eye Res. 2008. PMID: 18514644
-
Effects of bevacizumab (Avastin®) on the electroretinogram of isolated rat retina.Ophthalmic Res. 2011;46(3):145-51. doi: 10.1159/000324136. Epub 2011 Mar 4. Ophthalmic Res. 2011. PMID: 21372616
-
Safety aspects in the quality control of recombinant products from mammalian cell culture.J Pharm Biomed Anal. 1989;7(2):255-66. doi: 10.1016/0731-7085(89)80091-3. J Pharm Biomed Anal. 1989. PMID: 2488625 Review.
Cited by
-
Intravitreal anti-vascular endothelial growth factor monotherapy for large submacular hemorrhage secondary to neovascular age-related macular degeneration.Eye (Lond). 2015 Sep;29(9):1141-51. doi: 10.1038/eye.2015.131. Epub 2015 Aug 14. Eye (Lond). 2015. PMID: 26272443 Free PMC article.
-
[Prognosis and treatment of macular bleeding in neovascular age-related macular degeneration].Ophthalmologe. 2017 May;114(5):476-480. doi: 10.1007/s00347-017-0487-x. Ophthalmologe. 2017. PMID: 28405758 Review. German.
-
[Combined intravitreal injection of bevacizumab and SF6 gas for treatment of submacular hemorrhage secondary to age-related macular degeneration].Ophthalmologe. 2010 Apr;107(4):328-32. doi: 10.1007/s00347-009-2004-3. Ophthalmologe. 2010. PMID: 19669150 Clinical Trial. German.
-
[Intravitreal injection. Drugs to treat subretinal hemorrhage].Ophthalmologe. 2012 Jul;109(7):644-7. doi: 10.1007/s00347-012-2565-4. Ophthalmologe. 2012. PMID: 22752625 German.
-
Functional and structural outcome after vitrectomy combined with subretinal rtPA Injection with or without additional intravitreal Bevacizumab injection for submacular hemorrhages.PLoS One. 2021 Apr 30;16(4):e0250587. doi: 10.1371/journal.pone.0250587. eCollection 2021. PLoS One. 2021. PMID: 33930041 Free PMC article.
References
-
- Klein R, Klein B E, Linton K L. Prevalence of age‐related maculopathy. The Beaver Dam Eye Study. Ophthalmology 199299933–943. - PubMed
-
- Bressler N, Bressler S, Fine S. Age related macular degeneration. Surv Ophthalmol 198832375–413. - PubMed
-
- Berrocal M H, Lewis M L, Flynn H W., Jr Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol 1996122486–493. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources