Death don't have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity
- PMID: 17384171
- PMCID: PMC1867353
- DOI: 10.1105/tpc.106.045096
Death don't have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity
Abstract
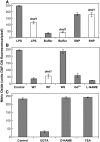
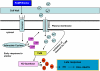
Plant innate immune response to pathogen infection includes an elegant signaling pathway leading to reactive oxygen species generation and resulting hypersensitive response (HR); localized programmed cell death in tissue surrounding the initial infection site limits pathogen spread. A veritable symphony of cytosolic signaling molecules (including Ca(2+), nitric oxide [NO], cyclic nucleotides, and calmodulin) have been suggested as early components of HR signaling. However, specific interactions among these cytosolic secondary messengers and their roles in the signal cascade are still unclear. Here, we report some aspects of how plants translate perception of a pathogen into a signal cascade leading to an innate immune response. We show that Arabidopsis thaliana CYCLIC NUCLEOTIDE GATED CHANNEL2 (CNGC2/DND1) conducts Ca(2+) into cells and provide a model linking this Ca(2+) current to downstream NO production. NO is a critical signaling molecule invoking plant innate immune response to pathogens. Plants without functional CNGC2 lack this cell membrane Ca(2+) current and do not display HR; providing the mutant with NO complements this phenotype. The bacterial pathogen-associated molecular pattern elicitor lipopolysaccharide activates a CNGC Ca(2+) current, which may be linked to NO generation due to buildup of cytosolic Ca(2+)/calmodulin.
Figures




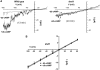


References
-
- Abramoff, M.D., Magelhaes, P.J., and Ram, S.J. (2004). Image processing with ImageJ. Biophotonics International 11 36–42.
-
- Ali, R., Zielinski, R., and Berkowitz, G.A. (2006). Expression of plant cyclic nucleotide-gated cation channels in yeast. J. Exp. Bot. 57 125–138. - PubMed
-
- Balagué, C., Lin, B., Alcon, C., Flottes, G., Malmström, S., Köhler, C., Neuhaus, G., Pelletier, G., Gaymard, F., and Roby, D. (2003). HLM1, and essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 15 365–379. - PMC - PubMed
-
- Bindschedler, L.V., Minibayeva, F., Gardner, S.L., Gerrish, C., Davies, D.R., and Bolwell, G.P. (2001). Early signalling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+. New Phytol. 151 185–194. - PubMed
-
- Bouché, N., Yellin, A., Snedden, W.A., and Fromm, H. (2005). Plant-specific calmodulin-binding proteins. Annu. Rev. Plant Biol. 56 435–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

