Roles of His-rich hpn and hpn-like proteins in Helicobacter pylori nickel physiology
- PMID: 17384182
- PMCID: PMC1913394
- DOI: 10.1128/JB.01245-06
Roles of His-rich hpn and hpn-like proteins in Helicobacter pylori nickel physiology
Abstract
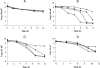
Individual gene-targeted hpn and hpn-like mutants and a mutant with mutations in both hpn genes were more sensitive to nickel, cobalt, and cadmium toxicity than was the parent strain, with the hpn-like strain showing the most metal sensitivity of the two individual His-rich protein mutants. The mutant strains contained up to eightfold more urease activity than the parent under nickel-deficient conditions, and the parent strain was able to achieve mutant strain activity levels by nickel supplementation. The mutants contained 3- to 4-fold more and the double mutant about 10-fold more Ni associated with their total urease pools, even though all of the strains expressed similar levels of total urease protein. Hydrogenase activities in the mutants were like those in the parent strain; thus, hydrogenase is fully activated under nickel-deficient conditions. The histidine-rich proteins appear to compete with the Ni-dependent urease maturation machinery under low-nickel conditions. Upon lowering the pH of the growth medium from 7.3 to 5, the wild-type urease activity increased threefold, but the activity in the three mutant strains was relatively unaffected. This pH effect was attributed to a nickel storage role for the His-rich proteins. Under low-nickel conditions, the addition of a nickel chelator did not significantly affect the urease activity of the wild type but decreased the activity of all of the mutants, supporting a role for the His-rich proteins as Ni reservoirs. These nickel reservoirs significantly impact the active urease activities achieved. The His-rich proteins play dual roles, as Ni storage and as metal detoxification proteins, depending on the exogenous nickel levels.
Figures

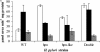
References
-
- Blaser, M. J. 1990. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect. Dis. 161:626-633. - PubMed
-
- Bury-Mone, S., J. M. Thiberge, M. Contreras, A. Maitournam, A. Labigne, and H. De Reuse. 2004. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori. Mol. Microbiol. 53:623-638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

