Microfluidic deletion/insertion analysis for rapid screening of KIT and PDGFRA mutations in CD117-positive gastrointestinal stromal tumors: diagnostic applications and report of a new KIT mutation
- PMID: 17384206
- PMCID: PMC1867442
- DOI: 10.2353/jmoldx.2007.060041
Microfluidic deletion/insertion analysis for rapid screening of KIT and PDGFRA mutations in CD117-positive gastrointestinal stromal tumors: diagnostic applications and report of a new KIT mutation
Abstract
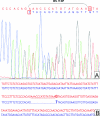
Gastrointestinal stromal tumors (GISTs) frequently harbor mutations in the KIT and PDGFRA genes, the presence and type of which correlate with the response to the kinase inhibitor imatinib mesylate. Because most GIST mutations are deletions/insertions, we used a microfluidic apparatus to detect these size variations in polymerase chain reaction-amplified DNA. This approach, termed microfluidic deletion/insertion analysis (MIDIA), identified mutations in 30 of 50 DNA samples from paraffin-embedded CD117-positive GISTs (60%), comprising 25 deletions and five insertions. Sequencing of 14 MIDIA-positive samples confirmed the deletions/insertions, including two 3-bp alterations. Sequencing of all 20 MIDIA-negative samples also showed highly consistent results with MIDIA because 10 cases were wild type and eight displayed a single base substitution in which detection by MIDIA was not expected. Sequencing also revealed a 3-bp deletion undetected by MIDIA, thus establishing the resolution limit of MIDIA at deletions/insertions >or=3 bp. Denaturing high-pressure liquid chromatography analysis confirmed all mutations detected by MIDIA and sequencing. We pro-pose MIDIA as the first step in mutational screening of GIST because it allowed the detection of 75% of mutated cases (94% of deletions/insertions) in less than 30 minutes after polymerase chain reaction amplification and at a lower cost compared with denaturing high-pressure liquid chromatography and sequencing, which might then be used only for MIDIA-negative cases.
Figures

Similar articles
-
Detection of c-KIT and PDGFRA gene mutations in gastrointestinal stromal tumors: comparison of DHPLC and DNA sequencing methods using a single population-based cohort.Am J Clin Pathol. 2010 Jan;133(1):149-55. doi: 10.1309/AJCP1FNW7RGZFTYU. Am J Clin Pathol. 2010. PMID: 20023271
-
Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis.Gastroenterology. 2006 May;130(6):1573-81. doi: 10.1053/j.gastro.2006.01.043. Gastroenterology. 2006. PMID: 16697720
-
Diagnostic relevance of overexpressions of PKC-θ and DOG-1 and KIT/PDGFRA gene mutations in extragastrointestinal stromal tumors: a Korean six-centers study of 28 cases.Anticancer Res. 2012 Mar;32(3):923-37. Anticancer Res. 2012. PMID: 22399613
-
Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours.Histopathology. 2008 Sep;53(3):245-66. doi: 10.1111/j.1365-2559.2008.02977.x. Epub 2008 Feb 28. Histopathology. 2008. PMID: 18312355 Review.
-
Pathology of gastrointestinal stromal tumors.Pathol Int. 2006 Jan;56(1):1-9. doi: 10.1111/j.1440-1827.2006.01924.x. Pathol Int. 2006. PMID: 16398673 Review.
Cited by
-
Pancreatic endocrine tumours: mutational and immunohistochemical survey of protein kinases reveals alterations in targetable kinases in cancer cell lines and rare primaries.Ann Oncol. 2012 Jan;23(1):127-134. doi: 10.1093/annonc/mdr048. Epub 2011 Mar 29. Ann Oncol. 2012. PMID: 21447618 Free PMC article.
-
Mutational profiling of kinases in human tumours of pancreatic origin identifies candidate cancer genes in ductal and ampulla of vater carcinomas.PLoS One. 2010 Sep 8;5(9):e12653. doi: 10.1371/journal.pone.0012653. PLoS One. 2010. PMID: 20838624 Free PMC article.
-
Cost-effectiveness of precision medicine in gastrointestinal stromal tumor and gastric adenocarcinoma.J Gastrointest Oncol. 2017 Jun;8(3):513-523. doi: 10.21037/jgo.2016.04.03. J Gastrointest Oncol. 2017. PMID: 28736638 Free PMC article. Review.
-
Identification of c-kit gene mutations in primary adenoid cystic carcinoma of the salivary gland.Mod Pathol. 2009 Oct;22(10):1296-302. doi: 10.1038/modpathol.2009.95. Epub 2009 Jul 17. Mod Pathol. 2009. PMID: 19617878 Free PMC article.
-
Molecular spectrum of c-KIT and PDGFRA gene mutations in gastro intestinal stromal tumor: determination of frequency, distribution pattern and identification of novel mutations in Indian patients.Med Oncol. 2015 Jan;32(1):424. doi: 10.1007/s12032-014-0424-7. Epub 2014 Dec 7. Med Oncol. 2015. PMID: 25481675
References
-
- Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer. 2005;103:821–829. - PubMed
-
- Tsujimura T, Makiishi-Shimobayashi C, Lundkvist J, Lendahl U, Nakasho K, Sugihara A, Iwasaki T, Mano M, Yamada N, Yamashita K, Toyosaka A, Terada N. Expression of the intermediate filament nestin in gastrointestinal stromal tumors and interstitial cells of Cajal. Am J Pathol. 2001;158:817–823. - PMC - PubMed
-
- Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H, Le Cesne A, McClure J, Maurel J, Nupponen N, Ray-Coquard I, Reichardt P, Sciot R, Stroobants S, van Glabbeke M, van Oosterom A, Demetri GD, GIST Consensus Meeting Panelists Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

