The S2 subsites of cathepsins K and L and their contribution to collagen degradation
- PMID: 17384231
- PMCID: PMC2203344
- DOI: 10.1110/ps.062666607
The S2 subsites of cathepsins K and L and their contribution to collagen degradation
Abstract
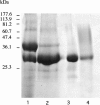
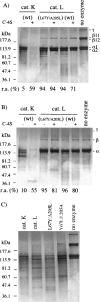
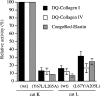
The exchange of residues 67 and 205 of the S2 pocket of human cysteine cathepsins K and L induces a permutation of their substrate specificity toward fluorogenic peptide substrates. While the cathepsin L-like cathepsin K (Tyr67Leu/Leu205Ala) mutant has a marked preference for Phe, the Leu67Tyr/Ala205Leu cathepsin L variant shows an effective cathepsin K-like preference for Leu and Pro. A similar turnaround of inhibition was observed by using specific inhibitors of cathepsin K [1-(N-Benzyloxycarbonyl-leucyl)-5-(N-Boc-phenylalanyl-leucyl)carbohydrazide] and cathepsin L [N-(4-biphenylacetyl)-S-methylcysteine-(D)-Arg-Phe-beta-phenethylamide]. Molecular modeling studies indicated that mutations alter the character of both S2 and S3 subsites, while docking calculations were consistent with kinetics data. The cathepsin K-like cathepsin L was unable to mimic the collagen-degrading activity of cathepsin K against collagens I and II, DQ-collagens I and IV, and elastin-Congo Red. In summary, double mutations of the S2 pocket of cathepsins K (Y67L/L205A) and L (L67Y/A205L) induce a switch of their enzymatic specificity toward small selective inhibitors and peptidyl substrates, confirming the key role of residues 67 and 205. However, mutations in the S2 subsite pocket of cathepsin L alone without engineering of binding sites to chondroitin sulfate are not sufficient to generate a cathepsin K-like collagenase, emphasizing the pivotal role of the complex formation between glycosaminoglycans and cathepsin K for its unique collagenolytic activity.
Figures

Similar articles
-
Selective inhibition of the collagenolytic activity of human cathepsin K by altering its S2 subsite specificity.Biochemistry. 2002 Jul 2;41(26):8447-54. doi: 10.1021/bi025638x. Biochemistry. 2002. PMID: 12081494
-
Antimicrobial Peptide LL-37 Is Both a Substrate of Cathepsins S and K and a Selective Inhibitor of Cathepsin L.Biochemistry. 2015 May 5;54(17):2785-98. doi: 10.1021/acs.biochem.5b00231. Epub 2015 Apr 27. Biochemistry. 2015. PMID: 25884905
-
Peptidyl vinyl sulphones: a new class of potent and selective cysteine protease inhibitors: S2P2 specificity of human cathepsin O2 in comparison with cathepsins S and L.Biochem J. 1996 Apr 1;315 ( Pt 1)(Pt 1):85-9. doi: 10.1042/bj3150085. Biochem J. 1996. PMID: 8670136 Free PMC article.
-
The consequences of lysosomotropism on the design of selective cathepsin K inhibitors.Chembiochem. 2006 Oct;7(10):1525-35. doi: 10.1002/cbic.200600149. Chembiochem. 2006. PMID: 16921579 Review.
-
Non-covalent cathepsin K inhibitors for the treatment of osteoporosis.Curr Top Med Chem. 2006;6(4):355-60. doi: 10.2174/156802606776287036. Curr Top Med Chem. 2006. PMID: 16611147 Review.
Cited by
-
Predicting Diagnostic Potential of Cathepsin in Epithelial Ovarian Cancer: A Design Validated by Computational, Biophysical and Electrochemical Data.Biomolecules. 2021 Dec 30;12(1):53. doi: 10.3390/biom12010053. Biomolecules. 2021. PMID: 35053201 Free PMC article.
-
Cathepsin K inhibitors for osteoporosis and potential off-target effects.Expert Opin Investig Drugs. 2009 May;18(5):585-600. doi: 10.1517/13543780902832661. Expert Opin Investig Drugs. 2009. PMID: 19388876 Free PMC article. Review.
-
The Unusual Resistance of Avian Defensin AvBD7 to Proteolytic Enzymes Preserves Its Antibacterial Activity.PLoS One. 2016 Aug 25;11(8):e0161573. doi: 10.1371/journal.pone.0161573. eCollection 2016. PLoS One. 2016. PMID: 27561012 Free PMC article.
-
Molecular and immunological characterization of cathepsin L-like cysteine protease of Paragonimus pseudoheterotremus.Parasitol Res. 2016 Dec;115(12):4457-4470. doi: 10.1007/s00436-016-5232-x. Epub 2016 Aug 26. Parasitol Res. 2016. PMID: 27562899
-
Dissecting the active site of the collagenolytic cathepsin L3 protease of the invasive stage of Fasciola hepatica.PLoS Negl Trop Dis. 2013 Jul 11;7(7):e2269. doi: 10.1371/journal.pntd.0002269. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23875031 Free PMC article.
References
-
- Aibe K., Yazawa, H., Abe, K., Teramura, K., Kumegawa, M., Kawashima, H., and Honda, K. 1996. Substrate specificity of recombinant osteoclast-specific cathepsin K from rabbits. Biol. Pharm. Bull. 19: 1026–1031. - PubMed
-
- Brömme D. and Kaleta, J. 2002. Thiol-dependent cathepsins: Pathophysiological implications and recent advances in inhibitor design. Curr. Pharm. Des. 8: 1639–1658. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

