Tight modulation of Escherichia coli bacterial biofilm formation through controlled expression of adhesion factors
- PMID: 17384304
- PMCID: PMC1907114
- DOI: 10.1128/AEM.02625-06
Tight modulation of Escherichia coli bacterial biofilm formation through controlled expression of adhesion factors
Abstract
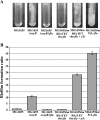
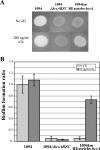
Despite the economic and sanitary problems caused by harmful biofilms, biofilms are nonetheless used empirically in industrial environmental and bioremediation processes and may be of potential use in medical settings for interfering with pathogen development. Escherichia coli is one of the bacteria with which biofilm formation has been studied in great detail, and it is especially appreciated for biotechnology applications because of its genetic amenability. Here we describe the development of two new genetic tools enabling the constitutive and inducible expression of any gene or operon of interest at its native locus. In addition to providing valuable tools for complementation and overexpression experiments, these two compact genetic cassettes were used to modulate the biofilm formation capacities of E. coli by taking control of two biofilm-promoting factors, autotransported antigen 43 adhesin and the bscABZC cellulose operon. The modulation of the biofilm formation capacities of E. coli or those of other bacteria capable of being genetically manipulated may be of use both for reducing and for improving the impact of biofilms in a number of industrial and medical applications.
Figures
Similar articles
-
Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene.J Bacteriol. 2001 Dec;183(24):7213-23. doi: 10.1128/JB.183.24.7213-7223.2001. J Bacteriol. 2001. PMID: 11717281 Free PMC article.
-
Combined inactivation and expression strategy to study gene function under physiological conditions: application to identification of new Escherichia coli adhesins.J Bacteriol. 2005 Feb;187(3):1001-13. doi: 10.1128/JB.187.3.1001-1013.2005. J Bacteriol. 2005. PMID: 15659678 Free PMC article.
-
Linkage between cellular adherence and biofilm formation in Escherichia coli O157:H7 EDL933.FEMS Microbiol Lett. 2011 Feb;315(1):46-53. doi: 10.1111/j.1574-6968.2010.02173.x. Epub 2010 Dec 17. FEMS Microbiol Lett. 2011. PMID: 21166710
-
"It's a gut feeling" - Escherichia coli biofilm formation in the gastrointestinal tract environment.Crit Rev Microbiol. 2018 Feb;44(1):1-30. doi: 10.1080/1040841X.2017.1303660. Epub 2017 May 9. Crit Rev Microbiol. 2018. PMID: 28485690 Review.
-
Acetate metabolism and Escherichia coli biofilm: new approaches to an old problem.FEMS Microbiol Lett. 2013 Jul;344(2):95-103. doi: 10.1111/1574-6968.12174. Epub 2013 Jun 3. FEMS Microbiol Lett. 2013. PMID: 23651469 Review.
Cited by
-
Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin.PLoS Genet. 2013;9(1):e1003144. doi: 10.1371/journal.pgen.1003144. Epub 2013 Jan 3. PLoS Genet. 2013. PMID: 23300476 Free PMC article.
-
YeeJ is an inverse autotransporter from Escherichia coli that binds to peptidoglycan and promotes biofilm formation.Sci Rep. 2017 Sep 12;7(1):11326. doi: 10.1038/s41598-017-10902-0. Sci Rep. 2017. PMID: 28900103 Free PMC article.
-
A new biofilm-associated colicin with increased efficiency against biofilm bacteria.ISME J. 2014 Jun;8(6):1275-88. doi: 10.1038/ismej.2013.238. Epub 2014 Jan 23. ISME J. 2014. PMID: 24451204 Free PMC article.
-
UpaG, a new member of the trimeric autotransporter family of adhesins in uropathogenic Escherichia coli.J Bacteriol. 2008 Jun;190(12):4147-61. doi: 10.1128/JB.00122-08. Epub 2008 Apr 18. J Bacteriol. 2008. PMID: 18424525 Free PMC article.
-
Discovery of New Genes Involved in Curli Production by a Uropathogenic Escherichia coli Strain from the Highly Virulent O45:K1:H7 Lineage.mBio. 2018 Aug 21;9(4):e01462-18. doi: 10.1128/mBio.01462-18. mBio. 2018. PMID: 30131362 Free PMC article.
References
-
- Aouad, G., J. L. Crovisier, V. A. Geoffroy, J. M. Meyer, and P. Stille. 2006. Microbially-mediated glass dissolution and sorption of metals by Pseudomonas aeruginosa cells and biofilm. J. Hazard. Mater. 136:889-895. - PubMed
-
- Beech, I. B., J. A. Sunner, and K. Hiraoka. 2005. Microbe-surface interactions in biofouling and biocorrosion processes. Int. Microbiol. 8:157-168. - PubMed
-
- Beloin, C., S. Da Re, and J.-M. Ghigo. August 2005, posting date. Chapter 8.3.1.3, Colonization of abiotic surfaces. In R. Curtiss III, A. Böck, J. L. Ingraham, J. B. Kaper, F. C. Neidhardt, M. Riley, and C. L. Squires (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org.
-
- Beloin, C., J. Valle, P. Latour-Lambert, P. Faure, M. Kzreminski, D. Balestrino, J. A. Haagensen, S. Molin, G. Prensier, B. Arbeille, and J.-M. Ghigo. 2004. Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Mol. Microbiol. 51:659-674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

