Inflammation in prostate carcinogenesis
- PMID: 17384581
- PMCID: PMC3552388
- DOI: 10.1038/nrc2090
Inflammation in prostate carcinogenesis
Abstract
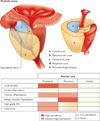
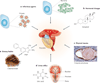
About 20% of all human cancers are caused by chronic infection or chronic inflammatory states. Recently, a new hypothesis has been proposed for prostate carcinogenesis. It proposes that exposure to environmental factors such as infectious agents and dietary carcinogens, and hormonal imbalances lead to injury of the prostate and to the development of chronic inflammation and regenerative 'risk factor' lesions, referred to as proliferative inflammatory atrophy (PIA). By developing new experimental animal models coupled with classical epidemiological studies, genetic epidemiological studies and molecular pathological approaches, we should be able to determine whether prostate cancer is driven by inflammation, and if so, to develop new strategies to prevent the disease.
Figures

References
-
- Jemal A, et al. Cancer statistics, 2005. CA Cancer J. Clin. 2005;55:10–30. - PubMed
-
-
Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc. Natl Acad. Sci. USA. 1995;92:5258–5265. This paper describes the main environmental causes of cancer and the molecular mechanisms by which they function.
-
-
- ACS. Cancer Facts and FIGS 2005. American Cancer Society. 2005:1–64.
-
- De Marzo AM, et al. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J. Cell Biochem. 2004;91:459–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

