Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation
- PMID: 17384649
- PMCID: PMC3427780
- DOI: 10.1038/nm1564
Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation
Abstract
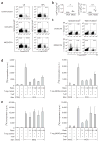
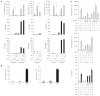
Treatment with ex vivo-generated regulatory T cells (T-reg) has been regarded as a potentially attractive therapeutic approach for autoimmune diseases. However, the dynamics and function of T-reg in autoimmunity are not well understood. Thus, we developed Foxp3gfp knock-in (Foxp3gfp.KI) mice and myelin oligodendrocyte glycoprotein (MOG)(35-55)/IA(b) (MHC class II) tetramers to track autoantigen-specific effector T cells (T-eff) and T-reg in vivo during experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis. MOG tetramer-reactive, Foxp3(+) T-reg expanded in the peripheral lymphoid compartment and readily accumulated in the central nervous system (CNS), but did not prevent the onset of disease. Foxp3(+) T cells isolated from the CNS were effective in suppressing naive MOG-specific T cells, but failed to control CNS-derived encephalitogenic T-eff that secreted interleukin (IL)-6 and tumor necrosis factor (TNF). Our data suggest that in order for CD4(+)Foxp3(+) T-reg to effectively control autoimmune reactions in the target organ, it may also be necessary to control tissue inflammation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Comment in
-
T effectors outfox T regulators in autoimmunity.Nat Med. 2007 Apr;13(4):411-3. doi: 10.1038/nm0407-411. Nat Med. 2007. PMID: 17415374 No abstract available.
References
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
-
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. - PubMed
-
- Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1P01NS38037-04/NS/NINDS NIH HHS/United States
- 2R01NS35685-06/NS/NINDS NIH HHS/United States
- R01 NS045937/NS/NINDS NIH HHS/United States
- R01 AI044880/AI/NIAID NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R37 NS030843/NS/NINDS NIH HHS/United States
- 2P01A139671-07/PHS HHS/United States
- R01 NS035685/NS/NINDS NIH HHS/United States
- 2R37NS30843-11/NS/NINDS NIH HHS/United States
- 1R01A144880-03/PHS HHS/United States
- 1R01NS045937-01/NS/NINDS NIH HHS/United States
- R01 NS046414/NS/NINDS NIH HHS/United States
- 1R01NS046414/NS/NINDS NIH HHS/United States
- P01 NS038037/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

