Fibroblast growth factor-hedgehog interdependence during retina regeneration
- PMID: 17385725
- PMCID: PMC2587111
- DOI: 10.1002/dvdy.21115
Fibroblast growth factor-hedgehog interdependence during retina regeneration
Abstract
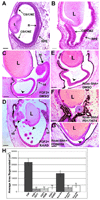
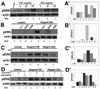
The embryonic chick is able to regenerate the retina after it has been removed. We have previously shown that proliferating stem/progenitor cells present in the ciliary body/ciliary marginal zone (CB/CMZ) of the chick eye are responsible for regeneration, which can be induced by ectopic fibroblast growth factor-2 (FGF2) or Sonic hedgehog (Shh). Here, we reveal the mechanisms showing how FGF2 and Shh signaling are interdependent during retina regeneration. If the FGF pathway is inhibited, regeneration stimulated by Shh is inhibited. Likewise, if the Hedgehog pathway is inhibited, regeneration stimulated by FGF2 is inhibited. We examined early signaling events in the CB/CMZ and found that FGF2 or Shh induced a robust Erk phosphorylation during the early stages of retina regeneration. Shh also up-regulated the expression of several members of the FGF signaling pathway. We show that ectopic FGF2 or Shh overexpression increased the number of phosphohistone 3 (PH3)-positive cells in the CB/CMZ and inhibition of either pathway decreased the number of PH3-positive cells. Additionally, both FGF and Hh signaling are required for cell survival in the CB/CMZ, whereas Hh and not FGF signaling plays a role in maintaining the identity of the retinal progenitor population in this region. Combined, our results support a model where the FGF and Hedgehog pathways work together to stimulate retina regeneration.
(c) 2007 Wiley-Liss, Inc.
Figures






Similar articles
-
The hedgehog pathway is a modulator of retina regeneration.Development. 2004 Sep;131(18):4607-21. doi: 10.1242/dev.01298. Development. 2004. PMID: 15342484
-
Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6.Mol Vis. 2007 Jan 24;13:57-65. Mol Vis. 2007. PMID: 17277739 Free PMC article.
-
Hedgehog signaling stimulates the formation of proliferating Müller glia-derived progenitor cells in the chick retina.Development. 2015 Aug 1;142(15):2610-22. doi: 10.1242/dev.121616. Epub 2015 Jun 26. Development. 2015. PMID: 26116667 Free PMC article.
-
The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye.Exp Eye Res. 2013 Nov;116:199-204. doi: 10.1016/j.exer.2013.08.018. Epub 2013 Sep 8. Exp Eye Res. 2013. PMID: 24025744 Review.
-
Cell signaling pathways in vertebrate lens regeneration.Curr Top Microbiol Immunol. 2013;367:75-98. doi: 10.1007/82_2012_289. Curr Top Microbiol Immunol. 2013. PMID: 23224710 Free PMC article. Review.
Cited by
-
Activin signaling limits the competence for retinal regeneration from the pigmented epithelium.Mech Dev. 2008 Jan-Feb;125(1-2):106-16. doi: 10.1016/j.mod.2007.10.001. Epub 2007 Oct 10. Mech Dev. 2008. PMID: 18042353 Free PMC article.
-
The chick eye in vision research: An excellent model for the study of ocular disease.Prog Retin Eye Res. 2017 Nov;61:72-97. doi: 10.1016/j.preteyeres.2017.06.004. Epub 2017 Jun 28. Prog Retin Eye Res. 2017. PMID: 28668352 Free PMC article. Review.
-
Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye.Cells. 2022 Nov 24;11(23):3755. doi: 10.3390/cells11233755. Cells. 2022. PMID: 36497013 Free PMC article. Review.
-
Pigment Epithelia of the Eye: Cell-Type Conversion in Regeneration and Disease.Life (Basel). 2022 Mar 6;12(3):382. doi: 10.3390/life12030382. Life (Basel). 2022. PMID: 35330132 Free PMC article. Review.
-
BMP signaling mediates stem/progenitor cell-induced retina regeneration.Proc Natl Acad Sci U S A. 2007 Dec 18;104(51):20380-5. doi: 10.1073/pnas.0708202104. Proc Natl Acad Sci U S A. 2007. PMID: 18093961 Free PMC article.
References
-
- Agius E, Soukkarieh C, Danesin C, Kan P, Takebayashi H, Soula C, Cochard P. Converse control of oligodendrocyte and astrocyte lineage development by Sonic hedgehog in the chick spinal cord. Dev. Biol. 2004;270(2):308–321. - PubMed
-
- Belecky-Adams T, Tomarev S, Li HS, Ploder L, McInnes RR, Sundin O, Adler R. Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Inves.t Ophthalmol. Vis. Sci. 1997;38(7):1293–1303. - PubMed
-
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101(4):435–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

