In vitro treatment of monocytes with 8-methoxypsolaren and ultraviolet A light induces dendritic cells with a tolerogenic phenotype
- PMID: 17386076
- PMCID: PMC1941926
- DOI: 10.1111/j.1365-2249.2007.03372.x
In vitro treatment of monocytes with 8-methoxypsolaren and ultraviolet A light induces dendritic cells with a tolerogenic phenotype
Abstract
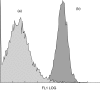
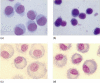
Extracorporeal photopheresis (ECP) has been considered an efficient dendritic cell (DC) therapy, used for treating both T cell malignancy, as well as T cell-mediated diseases. During the ECP procedure leucocytes are exposed to photoactivable agent 8-methoxypsolaren (8-MOP) and ultraviolet (UV) A radiation (PUVA) prior to reinfusion. Despite its clinical efficacy the mechanism of action remains elusive. As it has been reported that ECP might promote the differentiation of monocytes into immature DCs, we investigated the effects of UVA light (2 J/cm(2)) and 8-MOP (100 ng/ml) on in vitro monocyte-to-DC differentiation from normal donors. DCs were generated from human purified CD14(+) cells. Because monocytes are killed by PUVA and taking into account that only 5-10% of circulating mononuclear cells are exposed to PUVA during the ECP procedure, we developed an assay in which 10% of PUVA-treated monocytes were co-cultured with untreated monocytes. We first demonstrate that the presence of 10% apoptotic cells and monocyte activation were not enough to induce monocyte differentiation into DCs. Adding cytokines to our culture system, we obtained immature DCs characterized by significantly higher phagocytic activity and human leucocyte antigen D-related (HLA-DR) expression. These DCs preserved the capacity to be activated by lipopolysaccharide, but showed a reduced capacity to induce allogeneic T cell proliferation when first co-cultured with 10% of PUVA-treated cells. Our experimental design provides a novel insight into the real action of 8-MOP and UVA light on dendritic cell biology, suggesting an additional mechanism by which 8-MOP and UVA light exposure may influence immune responses.
Figures
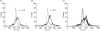
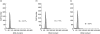
References
-
- Ardavin C, Amigorena S, Reis e Sousa C. Dendritic cells: immunobiology and cancer immunotherapy. Immunity. 2004;20:17–23. - PubMed
-
- Usharauli D. Dendritic cells and the immunity/tolerance decision. Med Hypoth. 2005;64:112–13. - PubMed
-
- Xiao BG, Huang YM, Link H. Dendritic cell vaccine design: strategies for eliciting peripheral tolerance as therapy of autoimmune diseases. Biodrugs. 2003;17:103–11. - PubMed
-
- Schlichting CL, Schareck WD, Nickel T, Weis M. Dendritic cells as pharmacological targets for the generation of regulatory immunosuppressive effectors. New implications for allo-transplantation. Curr Med Chem. 2005;12:1921–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

