Peroxisome proliferator-activated receptor gamma1 expression is diminished in human osteoarthritic cartilage and is downregulated by interleukin-1beta in articular chondrocytes
- PMID: 17386086
- PMCID: PMC1906809
- DOI: 10.1186/ar2151
Peroxisome proliferator-activated receptor gamma1 expression is diminished in human osteoarthritic cartilage and is downregulated by interleukin-1beta in articular chondrocytes
Abstract
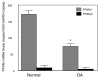
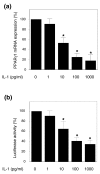
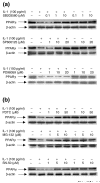
Peroxisome proliferator-activated receptor gamma (PPARgamma) is a nuclear receptor involved in the regulation of many cellular processes. We and others have previously shown that PPARgamma activators display anti-inflammatory and chondroprotective properties in vitro and improve the clinical course and histopathological features in an experimental animal model of osteoarthritis (OA). However, the expression and regulation of PPARgamma expression in cartilage are poorly defined. This study was undertaken to investigate the quantitative expression and distribution of PPARgamma in normal and OA cartilage and to evaluate the effect of IL-1beta, a prominent cytokine in OA, on PPARgamma expression in cultured chondrocytes. Immunohistochemical analysis revealed that the levels of PPARgamma protein expression were significantly lower in OA cartilage than in normal cartilage. Using real-time RT-PCR, we demonstrated that PPARgamma1 mRNA levels were about 10-fold higher than PPARgamma2 mRNA levels, and that only PPARgamma1 was differentially expressed: its levels in OA cartilage was 2.4-fold lower than in normal cartilage (p < 0.001). IL-1 treatment of OA chondrocytes downregulated PPARgamma1 expression in a dose- and time-dependent manner. This effect probably occurred at the transcriptional level, because IL-1 decreases both PPARgamma1 mRNA expression and PPARgamma1 promoter activity. TNF-alpha, IL-17, and prostaglandin E2 (PGE2), which are involved in the pathogenesis of OA, also downregulated PPARgamma1 expression. Specific inhibitors of the mitogen-activated protein kinases (MAPKs) p38 (SB203580) and c-Jun N-terminal kinase (SP600125), but not of extracellular signal-regulated kinase (PD98059), prevented IL-1-induced downregulation of PPARgamma1 expression. Similarly, inhibitors of NF-kappaB signaling (pyrrolidine dithiocarbamate, MG-132, and SN-50) abolished the suppressive effect of IL-1. Thus, our study demonstrated that PPARgamma1 is downregulated in OA cartilage. The pro-inflammatory cytokine IL-1 may be responsible for this downregulation via a mechanism involving activation of the MAPKs (p38 and JNK) and NF-kappaB signaling pathways. The IL-1-induced downregulation of PPARgamma expression might be a new and additional important process by which IL-1 promotes articular inflammation and cartilage degradation.
Figures



Similar articles
-
Advanced glycation end products downregulates peroxisome proliferator-activated receptor γ expression in cultured rabbit chondrocyte through MAPK pathway.Eur J Pharmacol. 2010 Dec 15;649(1-3):108-14. doi: 10.1016/j.ejphar.2010.09.025. Epub 2010 Sep 20. Eur J Pharmacol. 2010. PMID: 20863825
-
Mitogen-activated protein kinase and nuclear factor kappaB together regulate interleukin-17-induced nitric oxide production in human osteoarthritic chondrocytes: possible role of transactivating factor mitogen-activated protein kinase-activated proten kinase (MAPKAPK).Arthritis Rheum. 1999 Nov;42(11):2399-409. doi: 10.1002/1529-0131(199911)42:11<2399::AID-ANR19>3.0.CO;2-Y. Arthritis Rheum. 1999. PMID: 10555036
-
The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes.Arthritis Rheum. 2006 Jul;54(7):2152-63. doi: 10.1002/art.21951. Arthritis Rheum. 2006. PMID: 16802353
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
-
Biological actions of curcumin on articular chondrocytes.Osteoarthritis Cartilage. 2010 Feb;18(2):141-9. doi: 10.1016/j.joca.2009.10.002. Epub 2009 Oct 8. Osteoarthritis Cartilage. 2010. PMID: 19836480 Review.
Cited by
-
Activation of PPARs α, β/δ, and γ Impairs TGF-β1-Induced Collagens' Production and Modulates the TIMP-1/MMPs Balance in Three-Dimensional Cultured Chondrocytes.PPAR Res. 2010;2010:635912. doi: 10.1155/2010/635912. Epub 2010 Oct 4. PPAR Res. 2010. PMID: 20981144 Free PMC article.
-
PPARγ activation suppresses chondrocyte ferroptosis through mitophagy in osteoarthritis.J Orthop Surg Res. 2023 Aug 24;18(1):620. doi: 10.1186/s13018-023-04092-x. J Orthop Surg Res. 2023. PMID: 37620972 Free PMC article.
-
Roles of NF-kappaB activation and peroxisome proliferator-activated receptor gamma inhibition in the effect of rifampin on inducible nitric oxide synthase transcription in human lung epithelial cells.Antimicrob Agents Chemother. 2009 Apr;53(4):1539-45. doi: 10.1128/AAC.00961-08. Epub 2008 Dec 29. Antimicrob Agents Chemother. 2009. PMID: 19114679 Free PMC article.
-
PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes.Redox Biol. 2018 Apr;14:72-81. doi: 10.1016/j.redox.2017.08.011. Epub 2017 Aug 24. Redox Biol. 2018. PMID: 28869834 Free PMC article.
-
PPARγ/mTOR signalling: striking the right balance in cartilage homeostasis.Ann Rheum Dis. 2015 Mar;74(3):477-9. doi: 10.1136/annrheumdis-2014-206884. Epub 2015 Jan 14. Ann Rheum Dis. 2015. PMID: 25589512 Free PMC article. No abstract available.
References
-
- Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40:1–11. - PubMed
-
- Li X, Afif H, Cheng S, Martel-Pelletier J, Pelletier JP, Ranger P, Fahmi H. Expression and regulation of microsomal prostaglandin E synthase-1 in human osteoarthritic cartilage and chondrocytes. J Rheumatol. 2005;32:887–895. - PubMed
-
- Fahmi H, Pelletier JP, Martel-Pelletier J. PPARγ ligands as modulators of inflammatory and catabolic responses on arthritis. An overview. J Rheumatol. 2002;29:3–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

