Requirements for Cdk7 in the assembly of Cdk1/cyclin B and activation of Cdk2 revealed by chemical genetics in human cells
- PMID: 17386261
- PMCID: PMC1858677
- DOI: 10.1016/j.molcel.2007.02.003
Requirements for Cdk7 in the assembly of Cdk1/cyclin B and activation of Cdk2 revealed by chemical genetics in human cells
Abstract
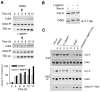
Cell division is controlled by cyclin-dependent kinases (CDKs). In metazoans, S phase onset coincides with activation of Cdk2, whereas Cdk1 triggers mitosis. Both Cdk1 and -2 require cyclin binding and T loop phosphorylation for full activity. The only known CDK-activating kinase (CAK) in metazoans is Cdk7, which is also part of the transcription machinery. To test the requirements for Cdk7 in vivo, we replaced wild-type Cdk7 with a version sensitive to bulky ATP analogs in human cancer cells. Selective inhibition of Cdk7 in G1 prevents activation (but not formation) of Cdk2/cyclin complexes and delays S phase. Inhibiting Cdk7 in G2 blocks entry to mitosis and disrupts Cdk1/cyclin B complex assembly, indicating that the two steps of Cdk1 activation-cyclin binding and T loop phosphorylation-are mutually dependent. Therefore, by combining chemical genetics and homologous gene replacement in somatic cells, we reveal different modes of CDK activation by Cdk7 at two distinct execution points in the cell cycle.
Figures




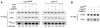

References
-
- Abbas T, Dutta A. CDK2-Activating Kinase (CAK): More Questions than Answers. Cell Cycle. 2006;5:1123–1124. - PubMed
-
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. - PubMed
-
- Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem. 2003;278:25752–25757. - PubMed
-
- Chen J, Larochelle S, Li X, Suter B. Xpd/Ercc2 regulates CAK activity and mitotic progression. Nature. 2003;424:228–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

