Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects
- PMID: 17387149
- PMCID: PMC1913270
- DOI: 10.1128/AAC.01181-06
Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects
Abstract
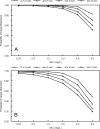
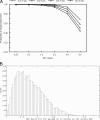
Ceftobiprole is a broad-spectrum cephalosporin with activity against methicillin-resistant Staphylococcus aureus (MRSA) that is undergoing phase III trials for the treatment of complicated skin and skin structure infections and nosocomial pneumonia. The objectives were to describe the pharmacodynamic profiles of ceftobiprole given at 500 mg intravenously (i.v.) every 8 h (q8h) (2-h infusion) and 500 mg i.v. every 12 h (q12h) (1-h infusion) to determine the overall probability of target attainment (PTA) by weighting for the expected distributions of renal function in the populations of interests, to determine the PTA against representative pathogens encountered in clinical trials, and to determine the optimal renal dose adjustment for ceftobiprole at 500 mg i.v. q8h (2-h infusion). Data for a total of 150 subjects in phase I/II trials were analyzed by using the population pharmacokinetic modeling program BigNPOD (nonparametric optimal design). Monte Carlo simulation was performed with the ADAPT II program to estimate the PTA at which the free drug concentrations exceed the MIC for 30 to 60% of the dosing interval (30 to 60% fT > MIC). For ceftobiprole at 500 mg i.v. q12h, the probabilities of achieving 30% and 50% fT > MIC exceeded 90% for MICs < or =2 mg/liter and < or =1 mg/liter, respectively, For ceftobiprole at 500 mg i.v. q8h, the probabilities of achieving 40 and 60% fT > MIC exceeded 90% for MICs < or =4 mg/liter and < or =2 mg/liter, respectively. For ceftobiprole at both 500 mg i.v. q12h and 500 mg i.v. q8h, the probability of achieving a nearly bactericidal effect (50% fT > MIC) exceeded 90% for methicillin-susceptible S. aureus and MRSA. For gram-negative pathogens, the PTA for achieving a nearly maximal bactericidal effect (60% fT > MIC) for ceftobiprole at 500 mg i.v. q8h exceeded 90% for non-AmpC-producing gram-negative organisms. Ceftobiprole at 500 mg i.v. q12h, for patients who had a creatinine clearance rate of < or =50 ml/min, was identified as the most appropriate treatment regimen for patients who require renal dose adjustment for mild to moderate renal impairment.
Figures

References
-
- Andes, D., and W. A. Craig. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 501376-1383. - PMC - PubMed
-
- Anonymous. 2004. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32470-485. - PubMed
-
- Bryskier, A. 2005. Anti-MRSA agents: under investigation, in the exploratory phase and clinically available. Expert Rev. Anti-Infect. Ther. 3505-553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

