Novel retinoic acid metabolism blocking agents have potent inhibitory activities on human breast cancer cells and tumour growth
- PMID: 17387344
- PMCID: PMC2360155
- DOI: 10.1038/sj.bjc.6603705
Novel retinoic acid metabolism blocking agents have potent inhibitory activities on human breast cancer cells and tumour growth
Abstract
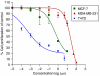
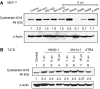
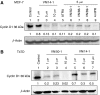
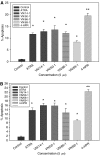
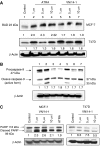
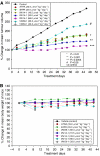
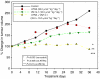
Antitumour effects of retinoids are attributed to their influence on cell proliferation, differentiation, apoptosis and angiogenesis. In our effort to develop useful agents for breast cancer therapy, we evaluated the effects of four representative retinoic acid metabolism blocking agents (RAMBAs, VN/14-1, VN/50-1, VN/66-1 and VN/69-1) on growth inhibition of oestrogen receptor positive (ER +ve, MCF-7 and T-47D) and oestrogen receptor negative (ER -ve, MDA-MB-231) human breast cancer cells. Additionally, we investigated the biological effects/molecular mechanism(s) underlying their growth inhibitory properties as well as their antitumour efficacies against MCF-7 and MCF-7Ca tumour xenografts in nude mice. We also assessed the effect of combining VN/14-1 and all-trans-retinoic acid (ATRA) on MCF-7 tumour xenografts. The ER +ve cell lines were more sensitive (IC(50) values between 3.0 and 609 nM) to the RAMBAs than the ER -ve MDA-MB-231 cell line (IC(50)=5.6-24.0 microM). Retinoic acid metabolism blocking agents induced cell differentiation as determined by increased expression of cytokeratin 8/18 and oestrogen receptor-alpha (ER-alpha). Similar to ATRA, they also induced apoptosis via activation of caspase 9. Cell cycle analysis indicated that RAMBAs arrested cells in the G1 and G2/M phases and caused significant downregulation (>80%) of cyclin D1 protein. In vivo, the growth of MCF-7 mammary tumours was dose-dependently and significantly inhibited (92.6%, P<0.0005) by VN/14-1. The combination of VN/14-1 and ATRA also inhibited MCF-7 breast tumour growth in vivo (up to 120%) as compared with single agents (P<0.025). VN/14-1 was also very effective in preventing the formation of MCF-7Ca tumours and it significantly inhibited the growth of established MCF-7Ca tumours, being as effective as the clinically used aromatase inhibitors, anastrozole and letrozole. Decrease in cyclin D1 and upregulation of cytokeratins, Bad and Bax with VN/14-1 may be responsible for the efficacy of this compound in inhibiting breast cancer cell growth in vitro and in vivo. Our results suggest that our RAMBAs, especially VN/14-1 may be useful novel therapy for breast cancer.
Figures
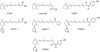

Similar articles
-
Effects of novel retinoic acid metabolism blocking agent (VN/14-1) on letrozole-insensitive breast cancer cells.Cancer Res. 2006 Dec 1;66(23):11485-93. doi: 10.1158/0008-5472.CAN-06-2168. Cancer Res. 2006. PMID: 17145897
-
Novel retinoic acid metabolism blocking agents endowed with multiple biological activities are efficient growth inhibitors of human breast and prostate cancer cells in vitro and a human breast tumor xenograft in nude mice.J Med Chem. 2004 Dec 30;47(27):6716-29. doi: 10.1021/jm0401457. J Med Chem. 2004. PMID: 15615521
-
Inhibitory effects of retinoic acid metabolism blocking agents (RAMBAs) on the growth of human prostate cancer cells and LNCaP prostate tumour xenografts in SCID mice.Br J Cancer. 2006 Feb 27;94(4):513-23. doi: 10.1038/sj.bjc.6602971. Br J Cancer. 2006. PMID: 16449997 Free PMC article.
-
Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases.Bioorg Med Chem. 2006 Jul 1;14(13):4323-40. doi: 10.1016/j.bmc.2006.02.041. Epub 2006 Mar 10. Bioorg Med Chem. 2006. PMID: 16530416 Review.
-
Cytochrome p450 retinoic acid 4-hydroxylase inhibitors: potential agents for cancer therapy.Mini Rev Med Chem. 2002 Jun;2(3):261-9. doi: 10.2174/1389557023406223. Mini Rev Med Chem. 2002. PMID: 12370067 Review.
Cited by
-
First chemical feature-based pharmacophore modeling of potent retinoidal retinoic acid metabolism blocking agents (RAMBAs): identification of novel RAMBA scaffolds.Eur J Med Chem. 2012 Jan;47(1):412-23. doi: 10.1016/j.ejmech.2011.11.010. Epub 2011 Nov 17. Eur J Med Chem. 2012. PMID: 22130607 Free PMC article.
-
Liposomal delivery of hydrophobic RAMBAs provides good bioavailability and significant enhancement of retinoic acid signalling in neuroblastoma tumour cells.J Drug Target. 2020 Jul;28(6):643-654. doi: 10.1080/1061186X.2019.1710157. Epub 2020 Jan 14. J Drug Target. 2020. PMID: 31903789 Free PMC article.
-
Therapeutic potential of the inhibition of the retinoic acid hydroxylases CYP26A1 and CYP26B1 by xenobiotics.Curr Top Med Chem. 2013;13(12):1402-28. doi: 10.2174/1568026611313120004. Curr Top Med Chem. 2013. PMID: 23688132 Free PMC article. Review.
-
Induction of miR-21 by retinoic acid in estrogen receptor-positive breast carcinoma cells: biological correlates and molecular targets.J Biol Chem. 2011 Feb 4;286(5):4027-42. doi: 10.1074/jbc.M110.184994. Epub 2010 Dec 3. J Biol Chem. 2011. PMID: 21131358 Free PMC article.
-
Intratumoural Cytochrome P450 Expression in Breast Cancer: Impact on Standard of Care Treatment and New Efforts to Develop Tumour-Selective Therapies.Biomedicines. 2021 Mar 12;9(3):290. doi: 10.3390/biomedicines9030290. Biomedicines. 2021. PMID: 33809117 Free PMC article. Review.
References
-
- Afonja O, Juste D, Das S, Matsuhashi S, Samuels HH (2004) Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene 23: 8135–8145 - PubMed
-
- Altucci L, Gronemeyer H (2001) The promise of retinoids to fight against cancer. Nat Rev Cancer 1: 181–193 - PubMed
-
- Belosay A, Brodie AMH, Njar VCO (2006) Effects of novel retinoic acid metabolism blocking agent (VN/14-1) on letrozole insensitive breast cancer cells. Cancer Res 66: 11485–11493 - PubMed
-
- Bollag W, Isnardi L, Jablonska S, Klaus M, Majewski S, Pirson W, Toma S (1997) Links between pharmacological properties of retinoids and nuclear retinoid receptors. Int J Cancer 70: 470–472 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

