Biomass in the manufacture of industrial products--the use of proteins and amino acids
- PMID: 17387469
- PMCID: PMC1914281
- DOI: 10.1007/s00253-007-0932-x
Biomass in the manufacture of industrial products--the use of proteins and amino acids
Abstract
The depletion in fossil feedstocks, increasing oil prices, and the ecological problems associated with CO2 emissions are forcing the development of alternative resources for energy, transport fuels, and chemicals: the replacement of fossil resources with CO2 neutral biomass. Allied with this, the conversion of crude oil products utilizes primary products (ethylene, etc.) and their conversion to either materials or (functional) chemicals with the aid of co-reagents such as ammonia and various process steps to introduce functionalities such as -NH2 into the simple structures of the primary products. Conversely, many products found in biomass often contain functionalities. Therefore, it is attractive to exploit this to bypass the use, and preparation of, co-reagents as well as eliminating various process steps by utilizing suitable biomass-based precursors for the production of chemicals. It is the aim of this mini-review to describe the scope of the possibilities to generate current functionalized chemical materials using amino acids from biomass instead of fossil resources, thereby taking advantage of the biomass structure in a more efficient way than solely utilizing biomass for the production of fuels or electricity.
Figures



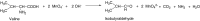

Similar articles
-
Bio-refinery as the bio-inspired process to bulk chemicals.Macromol Biosci. 2007 Feb 12;7(2):105-17. doi: 10.1002/mabi.200600223. Macromol Biosci. 2007. PMID: 17295397
-
Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change.Environ Sci Technol. 2007 Nov 15;41(22):7915-21. doi: 10.1021/es062559q. Environ Sci Technol. 2007. PMID: 18075108
-
Top chemical opportunities from carbohydrate biomass: a chemist's view of the Biorefinery.Top Curr Chem. 2014;353:1-40. doi: 10.1007/128_2014_544. Top Curr Chem. 2014. PMID: 24842622 Review.
-
Renewable resources in the chemical industry--breaking away from oil?Biotechnol J. 2007 Dec;2(12):1505-13. doi: 10.1002/biot.200700132. Biotechnol J. 2007. PMID: 17979162
-
Expanding the Boundary of Biorefinery: Organonitrogen Chemicals from Biomass.Acc Chem Res. 2021 Apr 6;54(7):1711-1722. doi: 10.1021/acs.accounts.0c00842. Epub 2021 Feb 12. Acc Chem Res. 2021. PMID: 33576600 Review.
Cited by
-
Biorefining of protein waste for production of sustainable fuels and chemicals.Biotechnol Biofuels. 2018 Sep 20;11:256. doi: 10.1186/s13068-018-1234-5. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 30250508 Free PMC article. Review.
-
Amino Acid Leaching of Critical Metals from Spent Lithium-Ion Batteries Followed by Selective Recovery of Cobalt Using Aqueous Biphasic System.ACS Omega. 2023 Jan 9;8(3):3198-3206. doi: 10.1021/acsomega.2c06654. eCollection 2023 Jan 24. ACS Omega. 2023. PMID: 36713728 Free PMC article.
-
Anti-inflammatory activity of palmitoylethanolamide ameliorates osteoarthritis induced by monosodium iodoacetate in Sprague-Dawley rats.Inflammopharmacology. 2021 Oct;29(5):1475-1486. doi: 10.1007/s10787-021-00870-3. Epub 2021 Sep 1. Inflammopharmacology. 2021. PMID: 34468900 Free PMC article.
-
The protein fraction from wheat-based dried distiller's grain with solubles (DDGS): extraction and valorization.N Biotechnol. 2015 Dec 25;32(6):606-11. doi: 10.1016/j.nbt.2015.01.007. Epub 2015 Jan 30. N Biotechnol. 2015. PMID: 25644639 Free PMC article. Review.
-
Metabolic engineering of Saccharomyces cerevisiae for production of novel cyanophycins with an extended range of constituent amino acids.Appl Environ Microbiol. 2009 Jun;75(11):3437-46. doi: 10.1128/AEM.00383-09. Epub 2009 Apr 3. Appl Environ Microbiol. 2009. PMID: 19346356 Free PMC article.
References
-
- Abdulazizkhan LH, Kembhavi MR, Nandibevoor ST. Kinetic and mechanistic study of the oxidative deamination and decarboxylation of l-valine by alkaline permanganate. Monatsh Chem. 2000;131:739–748. doi: 10.1007/s007060070075. - DOI
-
- Albanese AA, Irby V, Frankston JE. The utilization of d-amino acids by man. III. Arginine. J Biol Chem. 1945;160:25–30. - PubMed
-
- Arisseto AP, Toledo MCF. Acrylamide in foods: a review. Braz J Food Technol. 2006;9:123–134.
-
- Ben-Bassat A, Sariaslani FS, Huang LL, Patnaik R, Lowe DJ (2005) Methods for the preparation of para-hydroxycinnamic acid and cinnamic acid at alkaline pH. WO20050260724 A1
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

