Loperamide therapy for acute diarrhea in children: systematic review and meta-analysis
- PMID: 17388664
- PMCID: PMC1831735
- DOI: 10.1371/journal.pmed.0040098
Loperamide therapy for acute diarrhea in children: systematic review and meta-analysis
Abstract
Background: Loperamide is widely used in adults for acute diarrhea. However, its use in children has been discouraged by the World Health Organization and the American Academy of Pediatrics owing to concerns over safety and efficacy in young children.
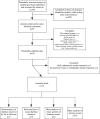
Methods and findings: To assess the efficacy and adverse effects of loperamide compared with placebo for acute diarrhea in children, we reviewed Medline, EMBase, the Cochrane Central Register of Controlled Trials, and bibliographies of known clinical trials and of review articles, and we also interviewed key investigators in the field. We undertook a systematic review and meta-analysis of randomized controlled trials of children younger than 12 y of age with acute diarrhea, comparing loperamide with placebo. Included trials reported data on diarrhea duration or severity, or provided data on adverse effects. Compared with patients who received placebo, patients allocated to loperamide were less likely to continue to have diarrhea at 24 h (prevalence ratio 0.66, 95% confidence interval [CI]: 0.57 to 0.78), had a shorter duration of diarrhea by 0.8 d (95% CI: 0.7 to 0.9 d), and had a lower count of stools at 24 h (0.84, 95% CI: 0.77 to 0.92). Results were similar when random-effects summaries were estimated. Serious adverse events, defined as ileus, lethargy, or death, were reported in eight out of 927 children allocated to loperamide (0.9%, 95% CI: 0.4% to 1.7%). Serious adverse events were not reported in any of the 764 children allocated to placebo (0%, 95% CI: 0% to 0.5%). Among the children allocated to loperamide, serious adverse events were reported only among children younger than 3 y.
Conclusions: In children who are younger than 3 y, malnourished, moderately or severely dehydrated, systemically ill, or have bloody diarrhea, adverse events outweigh benefits even at doses <or=0.25 mg/kg/d. In children who are older than 3 y with no/minimal dehydration, loperamide may be a useful adjunct to oral rehydration and early refeeding.
Conflict of interest statement
Figures




Comment in
-
Loperamide use for acute infectious diarrhea in children: safe and sound?Gastroenterology. 2008 Apr;134(4):1260-2. doi: 10.1053/j.gastro.2008.02.052. Gastroenterology. 2008. PMID: 18395108 No abstract available.
References
-
- Davidson G, Barnes G, Bass D, Cohen M, Fasano A, et al. Infectious diarrhea in children: Working Group Report of the First World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2002;35(Suppl 2):S143–S150. - PubMed
-
- Wingate D, Phillips SF, Lewis SJ, Malagelada JR, Speelman P, et al. Guidelines for adults on self-medication for the treatment of acute diarrhoea. Aliment Pharmacol Ther. 2001;15:773–782. - PubMed

