Extensive cortical remyelination in patients with chronic multiple sclerosis
- PMID: 17388943
- PMCID: PMC8095564
- DOI: 10.1111/j.1750-3639.2006.00043.x
Extensive cortical remyelination in patients with chronic multiple sclerosis
Abstract
Recent studies revealed prominent cortical demyelination in patients with chronic multiple sclerosis (MS). Demyelination in white matter lesions is frequently accompanied by remyelination. This repair process, however, often remains incomplete and restricted to the lesion border. In the present study, we examined the frequency and extent of remyelination in cortical and white matter lesions in autopsy brain tissue of 33 patients with chronic MS. The majority of patients (29 of 33) harbored cortical demyelination. Remyelination of cortical lesions was identified light microscopically by the presence of thin and irregularly arranged myelin sheaths, and confirmed by electron microscopy. Extensive remyelination was found in 18%, remyelination restricted to the lesion border in 54%, and no remyelination in 28% of cortical lesions. A direct comparison of the extent of remyelination in white matter and cortical lesions of the same patients revealed that remyelination of cortical lesions was consistently more extensive. In addition, g-ratios of fibers in areas of "normal appearing cortex" yielded values consistent with remyelination. Our data confirm the high prevalence of cortical demyelination in chronic MS and imply that the propensity to remyelinate is high in cortical MS lesions.
Figures


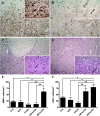

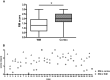
References
-
- Allen IV (1991) Pathology of multiple sclerosis. In: Mcalpine’s Multiple Sclerosis. Matthews WB (ed.), pp. 341–378. Churchill Livingstone: Edinburgh.
-
- Baracskay KL, Duchala CS, Miller RH, Macklin WB, Trapp BD (2002) Oligodendrogenesis is differentially regulated in gray and white matter of jimpy mice. J Neurosci Res 70:645–654. - PubMed
-
- Barkhof F, Bruck W, De Groot CJ, Bergers E, Hulshof S, Geurts J, Polman CH, Van Der Valk P (2003) Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch Neurol 60:1073–1081. - PubMed
-
- Barres BA, Raff MC (1993) Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361:258–260. - PubMed
-
- Blakemore WF (1973) Remyelination of the superior cerebellar peduncle in the mouse following demyelination induced by feeding cuprizone. J Neurol Sci 20:73–83. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

