Nodule formation and desmoplasia in medulloblastomas-defining the nodular/desmoplastic variant and its biological behavior
- PMID: 17388946
- PMCID: PMC8095556
- DOI: 10.1111/j.1750-3639.2007.00058.x
Nodule formation and desmoplasia in medulloblastomas-defining the nodular/desmoplastic variant and its biological behavior
Abstract
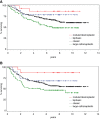
Among the variants of medulloblastoma in the current WHO classification of nervous system tumors, the desmoplastic variant, which has been reported to constitute 5%-25% of pediatric medulloblastomas, is defined by its nodular collections of neurocytic cells bounded by desmoplastic internodular zones. We have studied the frequency, morphological features and biological behavior of medulloblastomas in two contemporaneous SIOP/UKCCSG trial cohorts of children with medulloblastomas, CNS9102 (n = 315) and CNS9204 (n = 35), focusing on tumors with nodular and desmoplastic phenotypes. In children aged 3-16 years (CNS9102), the nodular/desmoplastic medulloblastoma represented 5% of all tumors, while in infants aged <3 years (CNS9204) this variant represented 57% of medulloblastomas. Using iFISH to detect molecular cytogenetic abnormalities in medulloblastomas with a nodular architecture, we demonstrated distinct genetic profiles in desmoplastic and non-desmoplastic (classic and anaplastic) tumors; in particular, abnormalities of chromosome 17 occurred in the latter, but not the former. Significantly different outcomes were demonstrated for classic, nodular/desmoplastic and large cell/anaplastic medulloblastomas in both cohorts. In conclusion, the nodular/desmoplastic medulloblastoma appears to have clinical, genetic and biological characteristics that set it apart from other variants of this tumor.
Figures
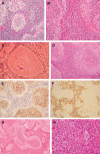


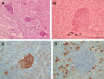
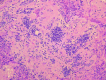
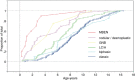
References
-
- Burger PC, Grahmann FC, Bliestle A, Kleihues P (1987) Differentiation in the medulloblastoma. A histological and immunohistochemical study. Acta Neuropathol (Berl) 73:115–123. - PubMed
-
- Burger PC, Scheithauer BW (1993) Tumors of the central nervous system, 3rd edn. Atlas of Tumor Pathology, Vol. 10. Rosai J (ed.), pp. 205–217. Armed Forces Insitute of Pathology: Washington.
-
- Chatty EM, Earle KM (1971) Medulloblastoma. A report of 201 cases with emphasis on the relationship of histologic variants to survival. Cancer 28:977–983. - PubMed
-
- Collett D (1996) Modelling Survival Data in Medical Research. Chapman & Hall: London.
-
- Eberhart CG, Brat DJ, Cohen KJ, Burger PC (2000) Pediatric neuroblastic brain tumors containing abundant neuropil and true rosettes. Pediatr Dev Pathol 3:346–352. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

