The role of the octarepeat region in neuroprotective function of the cellular prion protein
- PMID: 17388948
- PMCID: PMC1859984
- DOI: 10.1111/j.1750-3639.2007.00061.x
The role of the octarepeat region in neuroprotective function of the cellular prion protein
Abstract
Structural alterations of the cellular prion protein (PrP(C)) seem to be the core of the pathogenesis of prion diseases. However, the physiological function of PrP(C )remains an enigma. Cell culture experiments have indicated that PrP(C) and in particular its N-terminal octarepeat region together with the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways have a fundamental involvement in neuroprotection and oxidative stress reactions. We used wild-type mice, PrP knockout (Prnp(-/-)) animals and transgenic mice that lack the octarepeat region (C4/-) and subjected them to controlled ischemia. We identified an increased cleavage and synthesis of PrP(C) in ischemic brain areas of wild-type mice compared with sham controls. The infarct size in Prnp(-/-) animals was increased threefold when compared with wild-type mice. The infarct size in C4/- animals was identical to Prnp(-/-) mice, that is, around three times larger than in wild-type mice. We showed that the PrP in C4/- mice does not functionally rescue the Prnp(-/-) phenotype; furthermore it is unable to undergo beta cleavage, although an increased amount of C1 fragments was found in ischemic brain areas compared with sham controls. We demonstrated that the N-terminal octarepeat region has a lead function in PrP(C) physiology and neuroprotection against oxidative stress in vivo.
Figures
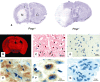



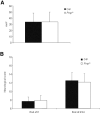

References
-
- Bounhar Y, Zhang Y, Goodyer CG, LeBlanc A (2001) Prion protein protects human neurons against Bax‐mediated apoptosis. J Biol Chem 276:39145–39149. - PubMed
-
- Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, Von Bohlen A, Schulz‐Schaeffer W, Giese A, Westaway D, Kretzschmar H (1997) The cellular prion protein binds copper in vivo. Nature 390:684–687. - PubMed
-
- Chen SG, Teplow DB, Parchi P, Teller JK, Gambetti P, Autilio‐Gambetti L (1995) Truncated forms of the human prion protein in normal brain and in prion diseases. J Biol Chem 270:19173–19180. - PubMed
-
- Drisaldi B, Coomaraswamy J, Mastrangelo P, Strome B, Yang J, Watts JC, Chishti MA, Marvi M, Windl O, Ahrens R, Major F, Sy MS, Kretzschmar H, Fraser PE, Mount HT, Westaway D (2004) Genetic mapping of activity determinants within cellular prion proteins: N‐terminal modules in PrPC offset pro‐apoptotic activity of the Doppel helix B/B′ region. J Biol Chem 279:55443–55454. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

