Effect of wine phenolics on cytokine-induced C-reactive protein expression
- PMID: 17388968
- PMCID: PMC2831220
- DOI: 10.1111/j.1538-7836.2007.02527.x
Effect of wine phenolics on cytokine-induced C-reactive protein expression
Abstract
Background: Elevation of C-reactive protein (CRP) levels in blood was recognized as one of the cardiac disease risk factors. Consumption of wine is shown to reduce the risk from heart disease and improve longevity.
Objectives: In the present study, we evaluated the effect of various wine polyphenolic compounds and several active synthetic derivatives of resveratrol on the inflammatory cytokines (IL-1beta + IL-6)-induced CRP expression in Hep3B cells.
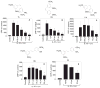
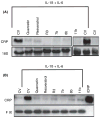
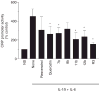
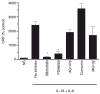
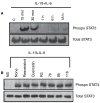
Results: Among the wine phenolics tested, quercetin and resveratrol, in a dose-dependent manner, suppressed cytokine-induced CRP expression. Two of the synthetic derivatives of resveratrol, R3 and 7b, elicited a fiftyfold higher suppressive effect compared with resveratrol. The inhibitory effects of resveratrol and its derivatives on CRP expression were at the level of mRNA production. Investigation of signaling pathways showed that the cytokines induced the phosphorylation of p38 and p44/42 MAP kinases. Inhibitors of p38 and p44/42 mitogen-activated protein kinase (MAPK) activation inhibited CRP expression, implicating the involvement of both pathways in cytokine-induced CRP expression. These data revealed a previously unrecognized role of the p44/42 MAPK signaling pathway in CRP expression. Wine polyphenolics or the synthetic compounds of resveratrol did not affect cytokine-activated phosphorylation of these MAPKs.
Conclusions: Wine phenolics inhibit CRP expression; however, to do so, they do not utilize the MAPK pathways.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures
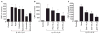
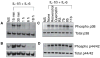
References
-
- Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279:48487–90. - PubMed
-
- Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–24. - PubMed
-
- Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–82. - PubMed
-
- Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004;44:6–11. - PubMed
-
- Verma S, Szmitko PE, Yeh ET. C-reactive protein: structure affects function. Circulation. 2004;109:1914–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

