Discovering structural motifs using a structural alphabet: application to magnesium-binding sites
- PMID: 17389049
- PMCID: PMC1851716
- DOI: 10.1186/1471-2105-8-106
Discovering structural motifs using a structural alphabet: application to magnesium-binding sites
Abstract
Background: For many metalloproteins, sequence motifs characteristic of metal-binding sites have not been found or are so short that they would not be expected to be metal-specific. Striking examples of such metalloproteins are those containing Mg2+, one of the most versatile metal cofactors in cellular biochemistry. Even when Mg2+-proteins share insufficient sequence homology to identify Mg2+-specific sequence motifs, they may still share similarity in the Mg2+-binding site structure. However, no structural motifs characteristic of Mg2+-binding sites have been reported. Thus, our aims are (i) to develop a general method for discovering structural patterns/motifs characteristic of ligand-binding sites, given the 3D protein structures, and (ii) to apply it to Mg2+-proteins sharing <30% sequence identity. Our motif discovery method employs structural alphabet encoding to convert 3D structures to the corresponding 1D structural letter sequences, where the Mg2+-structural motifs are identified as recurring structural patterns.
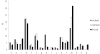
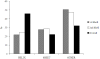
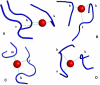
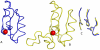
Results: The structural alphabet-based motif discovery method has revealed the structural preference of Mg2+-binding sites for certain local/secondary structures: compared to all residues in the Mg2+-proteins, both first and second-shell Mg2+-ligands prefer loops to helices. Even when the Mg2+-proteins share no significant sequence homology, some of them share a similar Mg2+-binding site structure: 4 Mg2+-structural motifs, comprising 21% of the binding sites, were found. In particular, one of the Mg2+-structural motifs found maps to a specific functional group, namely, hydrolases. Furthermore, 2 of the motifs were not found in non metalloproteins or in Ca2+-binding proteins. The structural motifs discovered thus capture some essential biochemical and/or evolutionary properties, and hence may be useful for discovering proteins where Mg2+ plays an important biological role.
Conclusion: The structural motif discovery method presented herein is general and can be applied to any set of proteins with known 3D structures. This new method is timely considering the increasing number of structures for proteins with unknown function that are being solved from structural genomics incentives. For such proteins, which share no significant sequence homology to proteins of known function, the presence of a structural motif that maps to a specific protein function in the structure would suggest likely active/binding sites and a particular biological function.
Figures


Similar articles
-
A structural-alphabet-based strategy for finding structural motifs across protein families.Nucleic Acids Res. 2010 Aug;38(14):e150. doi: 10.1093/nar/gkq478. Epub 2010 Jun 4. Nucleic Acids Res. 2010. PMID: 20525797 Free PMC article.
-
Magnesium and manganese binding sites on proteins have the same predominant motif of secondary structure.J Theor Biol. 2016 Apr 21;395:174-185. doi: 10.1016/j.jtbi.2016.02.006. Epub 2016 Feb 11. J Theor Biol. 2016. PMID: 26876751
-
Factors governing the substitution of La3+ for Ca2+ and Mg2+ in metalloproteins: a DFT/CDM study.J Am Chem Soc. 2005 Mar 23;127(11):4091-103. doi: 10.1021/ja044404t. J Am Chem Soc. 2005. PMID: 15771547
-
Structural characteristics of protein binding sites for calcium and lanthanide ions.J Biol Inorg Chem. 2001 Jun;6(5-6):479-89. doi: 10.1007/s007750100214. J Biol Inorg Chem. 2001. PMID: 11472012 Review.
-
Minimal Functional Sites in Metalloproteins and Their Usage in Structural Bioinformatics.Int J Mol Sci. 2016 May 4;17(5):671. doi: 10.3390/ijms17050671. Int J Mol Sci. 2016. PMID: 27153067 Free PMC article. Review.
Cited by
-
PTM-SD: a database of structurally resolved and annotated posttranslational modifications in proteins.Database (Oxford). 2014 May 24;2014:bau041. doi: 10.1093/database/bau041. Print 2014. Database (Oxford). 2014. PMID: 24857970 Free PMC article.
-
Local structural differences in homologous proteins: specificities in different SCOP classes.PLoS One. 2012;7(6):e38805. doi: 10.1371/journal.pone.0038805. Epub 2012 Jun 22. PLoS One. 2012. PMID: 22745680 Free PMC article.
-
A structural-alphabet-based strategy for finding structural motifs across protein families.Nucleic Acids Res. 2010 Aug;38(14):e150. doi: 10.1093/nar/gkq478. Epub 2010 Jun 4. Nucleic Acids Res. 2010. PMID: 20525797 Free PMC article.
-
Non-Conventional Metal Ion Cofactor Requirement of Dinoflagellate Alkaline Phosphatase and Translational Regulation by Phosphorus Limitation.Microorganisms. 2019 Aug 1;7(8):232. doi: 10.3390/microorganisms7080232. Microorganisms. 2019. PMID: 31374942 Free PMC article.
-
FINDSITE-metal: integrating evolutionary information and machine learning for structure-based metal-binding site prediction at the proteome level.Proteins. 2011 Mar;79(3):735-51. doi: 10.1002/prot.22913. Epub 2010 Dec 6. Proteins. 2011. PMID: 21287609 Free PMC article.
References
-
- Cowan JA. Biological Chemistry of Magnesium. New York , VCH; 1995.
-
- Dudev T, Cowan JA, Lim C. Competitive Binding in Magnesium Coordination Chemistry: Water versus Ligands of Biological Interest. J Am Chem Soc. 1999;121:7665–7673. doi: 10.1021/ja984470t. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

