Jagunal is required for reorganizing the endoplasmic reticulum during Drosophila oogenesis
- PMID: 17389229
- PMCID: PMC2064080
- DOI: 10.1083/jcb.200701048
Jagunal is required for reorganizing the endoplasmic reticulum during Drosophila oogenesis
Abstract
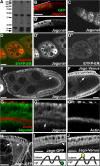
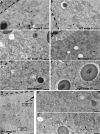
Vesicular traffic in the Drosophila melanogaster oocyte occurs actively during vitellogenesis. Although endocytosis in the oocyte has been well characterized, exocytic vesicular traffic is less well understood. We show that the oocyte endoplasmic reticulum (ER) becomes concentrated into subcortical clusters during vitellogenesis. This ER reorganization requires Jagunal, which is an evolutionarily conserved ER membrane protein. Loss of Jagunal reduces vesicular traffic to the oocyte lateral membrane, but does not affect posterior polarized vesicular traffic, suggesting a role for Jagunal in facilitating vesicular traffic in the subcortex. Reduced membrane traffic caused by loss of Jagunal affects oocyte and bristle growth. We propose that ER reorganization is an important mechanism used by cells to prepare for an increased demand for membrane traffic, and Jagunal facilitates this process through ER clustering.
Figures






Similar articles
-
The exocyst component Sec5 is present on endocytic vesicles in the oocyte of Drosophila melanogaster.J Cell Biol. 2005 Jun 20;169(6):953-63. doi: 10.1083/jcb.200411053. Epub 2005 Jun 13. J Cell Biol. 2005. PMID: 15955846 Free PMC article.
-
Sec61beta, a subunit of the Sec61 protein translocation channel at the endoplasmic reticulum, is involved in the transport of Gurken to the plasma membrane.BMC Cell Biol. 2009 Feb 18;10:11. doi: 10.1186/1471-2121-10-11. BMC Cell Biol. 2009. PMID: 19226464 Free PMC article.
-
Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells.J Cell Biol. 2005 May 23;169(4):635-46. doi: 10.1083/jcb.200410081. Epub 2005 May 16. J Cell Biol. 2005. PMID: 15897260 Free PMC article.
-
Gut thoughts on the Golgi complex.Traffic. 2000 Sep;1(9):738-45. doi: 10.1034/j.1600-0854.2000.010906.x. Traffic. 2000. PMID: 11208161 Review.
-
Bi-directional protein transport between the ER and Golgi.Annu Rev Cell Dev Biol. 2004;20:87-123. doi: 10.1146/annurev.cellbio.20.010403.105307. Annu Rev Cell Dev Biol. 2004. PMID: 15473836 Review.
Cited by
-
Drosophila sosie functions with β(H)-Spectrin and actin organizers in cell migration, epithelial morphogenesis and cortical stability.Biol Open. 2012 Oct 15;1(10):994-1005. doi: 10.1242/bio.20122154. Epub 2012 Aug 20. Biol Open. 2012. PMID: 23213377 Free PMC article.
-
Proteomic Analysis of Interaction between a Plant Virus and Its Vector Insect Reveals New Functions of Hemipteran Cuticular Protein.Mol Cell Proteomics. 2015 Aug;14(8):2229-42. doi: 10.1074/mcp.M114.046763. Epub 2015 Jun 19. Mol Cell Proteomics. 2015. PMID: 26091699 Free PMC article.
-
The endoplasmic reticulum is partitioned asymmetrically during mitosis before cell fate selection in proneuronal cells in the early Drosophila embryo.Mol Biol Cell. 2017 Jun 1;28(11):1530-1538. doi: 10.1091/mbc.E16-09-0690. Epub 2017 Apr 5. Mol Biol Cell. 2017. PMID: 28381427 Free PMC article.
-
Drosophila myt1 is the major cdk1 inhibitory kinase for wing imaginal disc development.Genetics. 2008 Dec;180(4):2123-33. doi: 10.1534/genetics.108.093195. Epub 2008 Oct 20. Genetics. 2008. PMID: 18940789 Free PMC article.
-
The apoptotic engulfment protein Ced-6 participates in clathrin-mediated yolk uptake in Drosophila egg chambers.Mol Biol Cell. 2012 May;23(9):1742-64. doi: 10.1091/mbc.E11-11-0939. Epub 2012 Mar 7. Mol Biol Cell. 2012. PMID: 22398720 Free PMC article.
References
-
- Ashburner, M. 1989. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
-
- Baumann, O., and B. Walz. 2001. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol. 205:149–214. - PubMed
-
- Bobinnec, Y., C. Marcaillou, X. Morin, and A. Debec. 2003. Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil. Cytoskeleton. 54:217–225. - PubMed
-
- Bretscher, M.S. 1996. Expression and changing distribution of the human transferrin receptor in developing Drosophila oocytes and embryos. J. Cell Sci. 109:3113–3119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

