Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2-deficient muscular dystrophy at all disease stages
- PMID: 17389231
- PMCID: PMC2064083
- DOI: 10.1083/jcb.200611152
Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2-deficient muscular dystrophy at all disease stages
Abstract
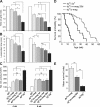
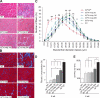
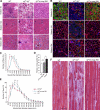
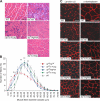
Mutations in laminin-alpha2 cause a severe congenital muscular dystrophy, called MDC1A. The two main receptors that interact with laminin-alpha2 are dystroglycan and alpha7beta1 integrin. We have previously shown in mouse models for MDC1A that muscle-specific overexpression of a miniaturized form of agrin (mini-agrin), which binds to dystroglycan but not to alpha7beta1 integrin, substantially ameliorates the disease (Moll, J., P. Barzaghi, S. Lin, G. Bezakova, H. Lochmuller, E. Engvall, U. Muller, and M.A. Ruegg. 2001. Nature. 413:302-307; Bentzinger, C.F., P. Barzaghi, S. Lin, and M.A. Ruegg. 2005. Matrix Biol. 24:326-332.). Now we show that late-onset expression of mini-agrin still prolongs life span and improves overall health, although not to the same extent as early expression. Furthermore, a chimeric protein containing the dystroglycan-binding domain of perlecan has the same activities as mini-agrin in ameliorating the disease. Finally, expression of full-length agrin also slows down the disease. These experiments are conceptual proof that linking the basement membrane to dystroglycan by specifically designed molecules or by endogenous ligands, could be a means to counteract MDC1A at a progressed stage of the disease, and thus opens new possibilities for the development of treatment options for this muscular dystrophy.
Figures




Similar articles
-
An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy.Nature. 2001 Sep 20;413(6853):302-7. doi: 10.1038/35095054. Nature. 2001. PMID: 11565031
-
Overexpression of mini-agrin in skeletal muscle increases muscle integrity and regenerative capacity in laminin-alpha2-deficient mice.FASEB J. 2005 Jun;19(8):934-42. doi: 10.1096/fj.04-3376com. FASEB J. 2005. PMID: 15923403
-
Laminin alpha1 chain improves laminin alpha2 chain deficient peripheral neuropathy.Hum Mol Genet. 2006 Sep 15;15(18):2690-700. doi: 10.1093/hmg/ddl201. Epub 2006 Aug 7. Hum Mol Genet. 2006. PMID: 16893907
-
The role of dystroglycan, a novel receptor of laminin and agrin, in cell differentiation.Histol Histopathol. 1997 Jan;12(1):195-203. Histol Histopathol. 1997. PMID: 9046055 Review.
-
Laminins in basement membrane assembly.Cell Adh Migr. 2013 Jan-Feb;7(1):56-63. doi: 10.4161/cam.21831. Epub 2012 Oct 17. Cell Adh Migr. 2013. PMID: 23076216 Free PMC article. Review.
Cited by
-
Whole Exome Sequencing as a Diagnostic Tool for Unidentified Muscular Dystrophy in a Vietnamese Family.Diagnostics (Basel). 2020 Sep 24;10(10):741. doi: 10.3390/diagnostics10100741. Diagnostics (Basel). 2020. PMID: 32987775 Free PMC article.
-
Dystroglycan and dystroglycanopathies: report of the 187th ENMC Workshop 11-13 November 2011, Naarden, The Netherlands.Neuromuscul Disord. 2012 Jul;22(7):659-68. doi: 10.1016/j.nmd.2012.02.006. Epub 2012 Mar 19. Neuromuscul Disord. 2012. PMID: 22437172 Free PMC article. No abstract available.
-
Multifunctionality of extracellular and cell surface heparan sulfate proteoglycans.Cell Mol Life Sci. 2009 Nov;66(21):3421-34. doi: 10.1007/s00018-009-0096-1. Epub 2009 Jul 24. Cell Mol Life Sci. 2009. PMID: 19629389 Free PMC article. Review.
-
Proinflammatory signals and the loss of lymphatic vessel hyaluronan receptor-1 (LYVE-1) in the early pathogenesis of laminin alpha2-deficient skeletal muscle.J Histochem Cytochem. 2011 Feb;59(2):167-79. doi: 10.1369/jhc.2010.956672. J Histochem Cytochem. 2011. PMID: 20876525 Free PMC article.
-
Skeletal muscle laminin and MDC1A: pathogenesis and treatment strategies.Skelet Muscle. 2011 Mar 1;1(1):9. doi: 10.1186/2044-5040-1-9. Skelet Muscle. 2011. PMID: 21798088 Free PMC article.
References
-
- Aumailley, M., L. Bruckner-Tuderman, W.G. Carter, R. Deutzmann, D. Edgar, P. Ekblom, J. Engel, E. Engvall, E. Hohenester, J.C. Jones, et al. 2005. A simplified laminin nomenclature. Matrix Biol. 24:326–332. - PubMed
-
- Bentzinger, C.F., P. Barzaghi, S. Lin, and M.A. Ruegg. 2005. Overexpression of mini-agrin in skeletal muscle increases muscle integrity and regenerative capacity in laminin-alpha2-deficient mice. FASEB J. 19:934–942. - PubMed
-
- Bezakova, G., and M.A. Ruegg. 2003. New insights into the roles of agrin. Nat. Rev. Mol. Cell Biol. 4:295–308. - PubMed
-
- Bogdanovich, S., T.O. Krag, E.R. Barton, L.D. Morris, L.A. Whittemore, R.S. Ahima, and T.S. Khurana. 2002. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 420:418–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

