Lymph node topology dictates T cell migration behavior
- PMID: 17389236
- PMCID: PMC2118562
- DOI: 10.1084/jem.20061278
Lymph node topology dictates T cell migration behavior
Abstract
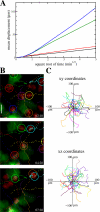
Adaptive immunity is initiated by T cell recognition of foreign peptides presented on dendritic cells (DCs) by major histocompatibility molecules. These interactions take place in secondary lymphoid tissues, such as lymph nodes (LNs) and spleen, and hence the anatomical structure of these tissues plays a crucial role in the development of immune responses. Two-photon microscopy (2PM) imaging in LNs suggests that T cells walk in a consistent direction for several minutes, pause briefly with a regular period, and then take off in a new, random direction. Here, we construct a spatially explicit model of T cell and DC migration in LNs and show that all dynamical properties of T cells could be a consequence of the densely packed LN environment. By means of 2PM experiments, we confirm that the large velocity fluctuations of T cells are indeed environmentally determined rather than resulting from an intrinsic motility program. Our simulations further predict that T cells self-organize into microscopically small, highly dynamic streams. We present experimental evidence for the presence of such turbulent streams in LNs. Finally, the model allows us to estimate the scanning rates of DCs (2,000 different T cells per hour) and T cells (100 different DCs per hour).
Figures




References
-
- Arstila, T.P., A. Casrouge, V. Baron, J. Even, J. Kanellopoulos, and P. Kourilsky. 1999. A direct estimate of the human αβ T cell receptor diversity. Science. 286:958–961. - PubMed
-
- Keşmir, C., J.A.M. Borghans, and R.J. de Boer. 2000. Diversity of human αβ T cell receptors. Science. 288:1135. - PubMed
-
- Miller, M.J., S.H. Wei, I. Parker, and M.D. Cahalan. 2002. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 296:1869–1873. - PubMed
-
- Bousso, P., N.R. Bhakta, R.S. Lewis, and E. Robey. 2002. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 296:1876–1880. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

