NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs
- PMID: 17389237
- PMCID: PMC2118560
- DOI: 10.1084/jem.20061293
NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs
Abstract
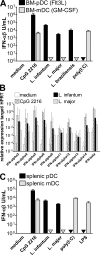
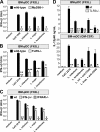
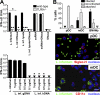
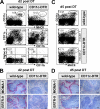
Natural killer (NK) cells are sentinel components of the innate response to pathogens, but the cell types, pathogen recognition receptors, and cytokines required for their activation in vivo are poorly defined. Here, we investigated the role of plasmacytoid dendritic cells (pDCs), myeloid DCs (mDCs), Toll-like receptors (TLRs), and of NK cell stimulatory cytokines for the induction of an NK cell response to the protozoan parasite Leishmania infantum. In vitro, pDCs did not endocytose Leishmania promastigotes but nevertheless released interferon (IFN)-alpha/beta and interleukin (IL)-12 in a TLR9-dependent manner. mDCs rapidly internalized Leishmania and, in the presence of TLR9, produced IL-12, but not IFN-alpha/beta. Depletion of pDCs did not impair the activation of NK cells in L. infantum-infected mice. In contrast, L. infantum-induced NK cell cytotoxicity and IFN-gamma production were abolished in mDC-depleted mice. The same phenotype was observed in TLR9(-/-) mice, which lacked IL-12 expression by mDCs, and in IL-12(-/-) mice, whereas IFN-alpha/beta receptor(-/-) mice showed only a minor reduction of NK cell IFN-gamma expression. This study provides the first direct evidence that mDCs are essential for eliciting NK cell cytotoxicity and IFN-gamma release in vivo and demonstrates that TLR9, mDCs, and IL-12 are functionally linked to the activation of NK cells in visceral leishmaniasis.
Figures




Similar articles
-
Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids.Immunity. 2007 Oct;27(4):597-609. doi: 10.1016/j.immuni.2007.08.017. Epub 2007 Oct 18. Immunity. 2007. PMID: 17950005
-
Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells.Proc Natl Acad Sci U S A. 2007 Jan 2;104(1):252-7. doi: 10.1073/pnas.0605978104. Epub 2006 Dec 26. Proc Natl Acad Sci U S A. 2007. PMID: 17190817 Free PMC article.
-
IL-18, but not IL-15, contributes to the IL-12-dependent induction of NK-cell effector functions by Leishmania infantum in vivo.Eur J Immunol. 2010 Jun;40(6):1708-17. doi: 10.1002/eji.200939988. Eur J Immunol. 2010. PMID: 20213736 Free PMC article.
-
Natural killer cell activation secondary to innate pattern sensing.J Innate Immun. 2011;3(3):264-73. doi: 10.1159/000326891. Epub 2011 Mar 29. J Innate Immun. 2011. PMID: 21454965 Review.
-
Effector and regulatory events during natural killer-dendritic cell interactions.Immunol Rev. 2006 Dec;214:219-28. doi: 10.1111/j.1600-065X.2006.00450.x. Immunol Rev. 2006. PMID: 17100887 Review.
Cited by
-
Leishmania interferes with host cell signaling to devise a survival strategy.J Biomed Biotechnol. 2010;2010:109189. doi: 10.1155/2010/109189. Epub 2010 Apr 8. J Biomed Biotechnol. 2010. PMID: 20396387 Free PMC article. Review.
-
Bacterial coinfection restrains antiviral CD8 T-cell response via LPS-induced inhibitory NK cells.Nat Commun. 2018 Oct 8;9(1):4117. doi: 10.1038/s41467-018-06609-z. Nat Commun. 2018. PMID: 30297690 Free PMC article.
-
Human lung cDC1 drive increased perforin-mediated NK cytotoxicity in chronic obstructive pulmonary disease.Am J Physiol Lung Cell Mol Physiol. 2021 Dec 1;321(6):L1183-L1193. doi: 10.1152/ajplung.00322.2020. Epub 2021 Oct 27. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34704847 Free PMC article.
-
Single-cell profiling of microenvironment components by spatial localization in pancreatic ductal adenocarcinoma.Theranostics. 2022 Jun 27;12(11):4980-4992. doi: 10.7150/thno.73222. eCollection 2022. Theranostics. 2022. PMID: 35836806 Free PMC article.
-
Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus Syrian hamster model.Vet Res. 2011 Feb 23;42(1):39. doi: 10.1186/1297-9716-42-39. Vet Res. 2011. PMID: 21345200 Free PMC article. Review.
References
-
- French, A.R., and W.M. Yokoyama. 2003. Natural killer cells and viral infections. Curr. Opin. Immunol. 15:45–51. - PubMed
-
- Korbel, D.S., O.C. Finney, and E.M. Riley. 2004. Natural killer cells and innate immunity to protozoan pathogens. Int. J. Parasitol. 34:1517–1528. - PubMed
-
- Martin-Fontecha, A., L.L. Thomsen, S. Brett, C. Gerard, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-γ for Th1 priming. Nat. Immunol. 5:1260–1265. - PubMed
-
- Laouar, Y., F.S. Sutterwala, L. Gorelik, and R.A. Flavell. 2005. Transforming growth factor-b controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat. Immunol. 6:600–607. - PubMed
-
- Moretta, A. 2002. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat. Rev. Immunol. 2:957–963. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

