The p23 molecular chaperone promotes functional telomerase complexes through DNA dissociation
- PMID: 17389357
- PMCID: PMC1851566
- DOI: 10.1073/pnas.0701442104
The p23 molecular chaperone promotes functional telomerase complexes through DNA dissociation
Abstract
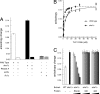
Telomeres are the composite of short DNA element tandem arrays and heterotypic protein components that protect and maintain chromosomal termini. As proper telomere maintenance requires a multitude of DNA extension events, it is important to understand the factors that modulate telomerase DNA association. Here, we show that the endogenous levels of the yeast p23 molecular chaperone Sba1p are required for telomere length maintenance and that Sba1p can modulate telomerase DNA binding and extension activities in vitro. Notably, telomere occupancy by telomerase and the extension rate of a shortened telomere fluctuated with changing Sba1 protein levels in vivo. In addition, we found that Sba1p displayed a cell cycle-dependent telomere interaction that paralleled telomerase binding; telomere association by Sba1p depended on its inherent chaperone activity. Taken together, our results support a model in which Sba1p modulates telomerase DNA binding activity for optimal function in vitro and in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




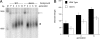
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

