Signaling-dependent and coordinated regulation of transcription, splicing, and translation resides in a single coregulator, PCBP1
- PMID: 17389360
- PMCID: PMC1851583
- DOI: 10.1073/pnas.0701065104
Signaling-dependent and coordinated regulation of transcription, splicing, and translation resides in a single coregulator, PCBP1
Abstract
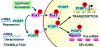
Transcription, splicing, and translation are potentially coordinately regulatable in a temporospatial-dependent manner, although supporting experimental evidence for this notion is scarce. Yeast two-hybrid screening of a mammary gland cDNA library with human p21-activated kinase 1 (Pak1) as bait identified polyC-RNA-binding protein 1 (PCBP1), which controls translation from mRNAs containing the DICE (differentiation control element). Mitogenic stimulation of human cells phosphorylated PCBP1 on threonines 60 and 127 in a Pak1-sensitive manner. Pak1-dependent phosphorylation of PCBP1 released its binding and translational inhibition from a DICE-minigene. Overexpression of PCBP1 also inhibited the translation of the endogenous L1 cell adhesion molecule mRNA, which contains two DICE motifs in the 3' untranslated region. We also found that Pak1 activation led to an increased nuclear retention of PCBP1, recruitment to the eukaryotic translation initiation factor 4E (eIF4E) promoter, and stimulation of eIF4E expression in a Pak1-sensitive manner. Moreover, mitogenic stimulation promoted Pak1- and PCBP1-dependent alternative splicing and exon inclusion from a CD44 minigene. The alternative splicing functions of PCBP1 were in turn mediated by its intrinsic interaction with Caper alpha, a U2 snRNP auxiliary factor-related protein previously implicated in RNA splicing. These findings establish the principle that a single coregulator can function as a signal-dependent and coordinated regulator of transcription, splicing, and translation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
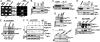

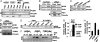
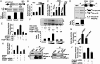
Similar articles
-
Protein 4.1R Exon 16 3' Splice Site Activation Requires Coordination among TIA1, Pcbp1, and RBM39 during Terminal Erythropoiesis.Mol Cell Biol. 2017 Apr 14;37(9):e00446-16. doi: 10.1128/MCB.00446-16. Print 2017 May 1. Mol Cell Biol. 2017. PMID: 28193846 Free PMC article.
-
THAP11, a novel binding protein of PCBP1, negatively regulates CD44 alternative splicing and cell invasion in a human hepatoma cell line.FEBS Lett. 2012 May 21;586(10):1431-8. doi: 10.1016/j.febslet.2012.04.016. Epub 2012 Apr 21. FEBS Lett. 2012. PMID: 22673507
-
Identification of gamma-synuclein as a new PCBP1-interacting protein.Neurol Res. 2016 Dec;38(12):1064-1078. doi: 10.1179/1743132815Y.0000000091. Epub 2016 Oct 27. Neurol Res. 2016. PMID: 26344801
-
Splicing factor poly(rC)-binding protein 1 is a novel and distinctive tumor suppressor.J Cell Physiol. 2018 Jan;234(1):33-41. doi: 10.1002/jcp.26873. Epub 2018 Aug 21. J Cell Physiol. 2018. PMID: 30132844 Review.
-
eIF4E, the mRNA cap-binding protein: from basic discovery to translational research.Biochem Cell Biol. 2008 Apr;86(2):178-83. doi: 10.1139/O08-034. Biochem Cell Biol. 2008. PMID: 18443631 Review.
Cited by
-
Poly(C)-binding protein 1, a novel N(pro)-interacting protein involved in classical swine fever virus growth.J Virol. 2013 Feb;87(4):2072-80. doi: 10.1128/JVI.02807-12. Epub 2012 Dec 5. J Virol. 2013. PMID: 23221550 Free PMC article.
-
Novel function of the poly(c)-binding protein α-CP2 as a transcriptional activator that binds to single-stranded DNA sequences.Int J Mol Med. 2013 Nov;32(5):1187-94. doi: 10.3892/ijmm.2013.1488. Epub 2013 Sep 11. Int J Mol Med. 2013. PMID: 24026233 Free PMC article.
-
Incrimination of heterogeneous nuclear ribonucleoprotein E1 (hnRNP-E1) as a candidate sensor of physiological folate deficiency.J Biol Chem. 2011 Nov 11;286(45):39100-15. doi: 10.1074/jbc.M111.230938. Epub 2011 Sep 19. J Biol Chem. 2011. PMID: 21930702 Free PMC article.
-
Current progress on innate immune evasion mediated by Npro protein of pestiviruses.Front Immunol. 2023 Apr 5;14:1136051. doi: 10.3389/fimmu.2023.1136051. eCollection 2023. Front Immunol. 2023. PMID: 37090696 Free PMC article. Review.
-
Evidence Favoring a Positive Feedback Loop for Physiologic Auto Upregulation of hnRNP-E1 during Prolonged Folate Deficiency in Human Placental Cells.J Nutr. 2017 Apr;147(4):482-498. doi: 10.3945/jn.116.241364. Epub 2017 Mar 1. J Nutr. 2017. PMID: 28250194 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

