In vivo negative selection screen identifies genes required for Francisella virulence
- PMID: 17389372
- PMCID: PMC1832217
- DOI: 10.1073/pnas.0609675104
In vivo negative selection screen identifies genes required for Francisella virulence
Abstract
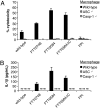
Francisella tularensis subverts the immune system to rapidly grow within mammalian hosts, often causing tularemia, a fatal disease. This pathogen targets the cytosol of macrophages where it replicates by using the genes encoded in the Francisella pathogenicity island. However, the bacteria are recognized in the cytosol by the host's ASC/caspase-1 pathway, which is essential for host defense, and leads to macrophage cell death and proinflammatory cytokine production. We used a microarray-based negative selection screen to identify Francisella genes that contribute to growth and/or survival in mice. The screen identified many known virulence factors including all of the Francisella pathogenicity island genes, LPS O-antigen synthetic genes, and capsule synthetic genes. We also identified 44 previously unidentified genes that were required for Francisella virulence in vivo, indicating that this pathogen may use uncharacterized mechanisms to cause disease. Among these, we discovered a class of Francisella virulence genes that are essential for growth and survival in vivo but do not play a role in intracellular replication within macrophages. Instead, these genes modulate the host ASC/caspase-1 pathway, a previously unidentified mechanism of Francisella pathogenesis. This finding indicates that the elucidation of the molecular mechanisms used by other uncharacterized genes identified in our screen will increase our understanding of the ways in which bacterial pathogens subvert the immune system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

