Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger
- PMID: 17389374
- PMCID: PMC1832220
- DOI: 10.1073/pnas.0609379104
Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger
Abstract
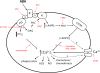
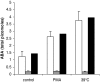
Abscisic acid (ABA) is a phytohormone involved in fundamental physiological processes of higher plants, such as response to abiotic stress (temperature, light, drought), regulation of seed dormancy and germination, and control of stomatal closure. Here, we provide evidence that ABA stimulates several functional activities [phagocytosis, reactive oxygen species and nitric oxide (NO) production, and chemotaxis] of human granulocytes through a signaling pathway sequentially involving a pertussis toxin (PTX)-sensitive G protein/receptor complex, protein kinase A activation, ADP-ribosyl cyclase phosphorylation, and consequent cyclic-ADP-ribose overproduction, leading to an increase of the intracellular Ca(2+) concentration. The increase of free intracellular ABA and its release by activated human granulocytes indicate that ABA should be considered as a new pro-inflammatory cytokine in humans. This discovery is an intriguing example of conservation of a hormone and its signaling pathway from plants to humans and provides insight into the molecular mechanisms of granulocyte activation, possibly leading to the development of new antiinflammatory drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
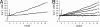
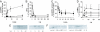
References
-
- Nambara E, Marion-Poll A. Annu Rev Plant Biol. 2005;56:165–185. - PubMed
-
- Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DL. J Biol Chem. 1989;264:1608–1615. - PubMed
-
- Wu Y, Kuzma J, Marechal E, Graeff R, Lee HC, Foster R, Chua NH. Science. 1997;278:2126–2130. - PubMed
-
- Puce S, Basile G, Bavestrello G, Bruzzone S, Cerrano C, Giovine M, Arillo A, Zocchi E. J Biol Chem. 2004;279:39783–39788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

