Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy
- PMID: 17389386
- PMCID: PMC1851611
- DOI: 10.1073/pnas.0700036104
Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy
Abstract
Mycobacterium tuberculosis parasitizes resting macrophages yet is killed by activated macrophages through both oxidative and nonoxidative mechanisms. Nonoxidative mechanisms are linked to the maturation of the bacteria-containing phagosome into an acidified, hydrolytically active compartment. We describe here a mechanism for killing Mycobacteria in the lysosomal compartment through the activity of peptides generated by the hydrolysis of ubiquitin. The induction of autophagy in infected macrophages enhanced the delivery of ubiquitin conjugates to the lysosome and increased the bactericidal capacity of the lysosomal soluble fraction. The accumulation of ubiquitinated proteins in the autophagolysosome provides one possible mechanism behind the antimicrobial activities observed for a range of pathogens in autophagous host cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
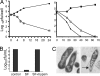

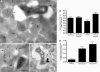
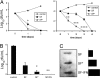

Comment in
-
Ubiquitin trafficking to the lysosome: keeping the house tidy and getting rid of unwanted guests.Autophagy. 2007 Jul-Aug;3(4):399-401. doi: 10.4161/auto.4272. Epub 2007 Jul 12. Autophagy. 2007. PMID: 17457035
References
-
- Deretic V, Singh S, Master S, Harris J, Roberts E, Kyei G, Davis A, de Haro S, Naylor J, Lee HH, Vergne I. Cell Microbiol. 2006;8:719–727. - PubMed
-
- Russell DG. Nat Rev Mol Cell Biol. 2001;2:569–577. - PubMed
-
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Science. 1994;263:678–681. - PubMed
-
- MacMicking JD, Taylor GA, McKinney JD. Science. 2003;302:654–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

