Suitability of recommended limits for fasting glucose tests in women with polycystic ovary syndrome
- PMID: 17389441
- PMCID: PMC1828174
- DOI: 10.1503/cmaj.060607
Suitability of recommended limits for fasting glucose tests in women with polycystic ovary syndrome
Abstract
Background: The Canadian and American Diabetes Associations recommend the use of an oral glucose tolerance test to screen for abnormal glucose tolerance among women with polycystic ovary syndrome when their fasting plasma glucose level is 5.7 mmol/L or more (Canadian guideline) and 5.6 mmol/L or more (American). Our objective was to determine the predictive value of 5.6 mmol/L as a fasting plasma glucose cutoff for detecting abnormal glucose tolerance in women with polycystic ovary syndrome, and then to define the optimal cutoff for this population.
Methods: An oral glucose tolerance test was administered to 105 consecutive women with polycystic ovary syndrome referred to an academic reproductive endocrine clinic. We calculated sensitivity, specificity and likelihood ratios.
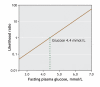
Results: The sensitivity of a 5.6 mmol/L cutoff was 48% (95% confidence interval [CI] 30%-67%); its specificity, 98.7% (95% CI 96.1%-100%). With this cutoff, 52% of women with polycystic ovary syndrome whose glucose tolerance is abnormal would be missed. The prevalence of abnormal glucose tolerance was 28%, with a positive predictive value of 93% (95% CI 81%-100%) and a negative predictive value of 83% (95% CI 76%-91%). The likelihood ratio for a positive test was 36.7 (95% CI 5.0-267), and for a negative test, 0.5 (95% CI 0.4-0.7). The optimal fasting plasma glucose cutoff value was 5.0 mmol/L, with a 79% sensitivity (95% CI 65%-94%) and 66% specificity (95% CI 55%-77%). If this cutoff were used, 24% of women with abnormal glucose tolerance would still be missed.
Interpretation: The Canadian and American recommendations--of screening for abnormal glucose tolerance with an oral glucose tolerance test only when the results of a fasting plasma glucose test are 5.7 mmol/L (or 5.6 mmol/L) or more--are inappropriate for women with polycystic ovary syndrome. We therefore recommend that all women with polycystic ovary syndrome have an oral glucose tolerance test.
Figures


Comment in
-
Screening for diabetes in women with polycystic ovary syndrome.CMAJ. 2007 Mar 27;176(7):951-2. doi: 10.1503/cmaj.070200. CMAJ. 2007. PMID: 17389444 Free PMC article. No abstract available.
Similar articles
-
A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome.J Clin Endocrinol Metab. 1998 Aug;83(8):2694-8. doi: 10.1210/jcem.83.8.5054. J Clin Endocrinol Metab. 1998. PMID: 9709933
-
The utility of fasting plasma glucose to identify impaired glucose metabolism in women with polycystic ovary syndrome.Gynecol Endocrinol. 2014 Sep;30(9):664-6. doi: 10.3109/09513590.2014.912265. Epub 2014 Apr 15. Gynecol Endocrinol. 2014. PMID: 24734869
-
Screening for diabetes in women with polycystic ovary syndrome.CMAJ. 2007 Mar 27;176(7):951-2. doi: 10.1503/cmaj.070200. CMAJ. 2007. PMID: 17389444 Free PMC article. No abstract available.
-
Diagnostic accuracy of oral glucose tolerance tests, fasting plasma glucose and haemoglobin A1c for type 2 diabetes in women with polycystic ovary syndrome: A systematic review and meta-analysis.Diabetes Metab Syndr. 2024 Mar;18(3):102970. doi: 10.1016/j.dsx.2024.102970. Epub 2024 Feb 28. Diabetes Metab Syndr. 2024. PMID: 38442646
-
Value of 1-Hour Plasma Glucose During an Oral Glucose Tolerance Test in a Multiethnic Cohort of Obese Children and Adolescents.Clin Med Insights Endocrinol Diabetes. 2023 Jun 9;16:11795514231177206. doi: 10.1177/11795514231177206. eCollection 2023. Clin Med Insights Endocrinol Diabetes. 2023. PMID: 37323220 Free PMC article. Review.
Cited by
-
Insulin resistance and adverse metabolic profile in overweight/obese and normal weight of young women with polycystic ovary syndrome.Caspian J Intern Med. 2018 Summer;9(3):260-267. doi: 10.22088/cjim.9.3.260. Caspian J Intern Med. 2018. PMID: 30197771 Free PMC article.
-
Diabetes and pregnancy: an endocrine society clinical practice guideline.J Clin Endocrinol Metab. 2013 Nov;98(11):4227-49. doi: 10.1210/jc.2013-2465. J Clin Endocrinol Metab. 2013. Update in: J Clin Endocrinol Metab. 2018 Nov 1;103(11):4042. doi: 10.1210/jc.2018-01939. PMID: 24194617 Free PMC article. Updated.
-
PCOS in adolescence and type 2 diabetes.Curr Diab Rep. 2015 Jan;15(1):564. doi: 10.1007/s11892-014-0564-3. Curr Diab Rep. 2015. PMID: 25398203 Review.
-
Effect of Vitex negundo L. seeds in letrozole induced polycystic ovarian syndrome.J Tradit Complement Med. 2018 Oct 11;9(4):336-345. doi: 10.1016/j.jtcme.2018.03.001. eCollection 2019 Oct. J Tradit Complement Med. 2018. PMID: 31453130 Free PMC article.
-
Insulin and hyperandrogenism in women with polycystic ovary syndrome.J Steroid Biochem Mol Biol. 2010 Oct;122(1-3):42-52. doi: 10.1016/j.jsbmb.2009.12.010. Epub 2009 Dec 28. J Steroid Biochem Mol Biol. 2010. PMID: 20036327 Free PMC article. Review.
References
-
- Asuncion M, Calvo RM, San Millan JL, et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 2000;85:2434-8. - PubMed
-
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 1999;84:4006-11. - PubMed
-
- Knochenhauer ES, Key TJ, Kahsar-Miller M, et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998;83:3078-82. - PubMed
-
- Toprak S, Yonem A, Cakir B, et al. Insulin resistance in nonobese patients with polycystic ovary syndrome. Horm Res 2001;55:65-70. - PubMed
-
- Ciampelli M, Fulghesu AM, Cucinelli F, et al. Heterogeneity in β cell activity, hepatic insulin clearance and peripheral insulin sensitivity in women with polycystic ovary syndrome. Hum Reprod 1997;12:1897-901. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
