Identification of novel dendritic cell populations in normal mouse retina
- PMID: 17389502
- PMCID: PMC2446435
- DOI: 10.1167/iovs.06-0697
Identification of novel dendritic cell populations in normal mouse retina
Abstract
Purpose: Whether tissue resident or infiltrating antigen-presenting cells (APCs) are involved in modulating immune responses in the retina and initiating inflammation is controversial. In this histologic study, the authors examine the retinas of mice strains with different susceptibility to experimental autoimmune uveoretinitis (EAU) for tissue resident APC.
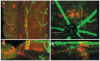
Methods: Retinal wholemounts from normal and inflamed eyes of B10R III, C57BL/6, BALB/c, and ABH Biozii mice were immunostained for APC markers (33D1, CD11c, CD11b, major histocompatibility complex [MHC] class II, F4/80, CD80, CD86, CD205, mPDCA, B220, and GR1) and analyzed by confocal fluorescence microscopy using emission fingerprinting and three-dimensional reconstruction techniques. Hematoxylin and eosin-stained histologic sections were used to evaluate EAU disease scores and to assess outer blood retina barrier (retinal pigment epithelium [RPE]) structures.
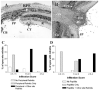
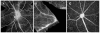
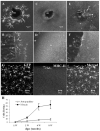
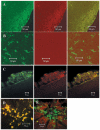
Results: A population of 33D1(+) cells was identified exclusively in the peripheral margins and juxtapapillary areas of the retina in normal, nonimmunized C57BL/6 adult mice. These cells were also MHC class II(high), and their location corresponded to sites of earliest inflammation in EAU. Numbers in the papillary area were very low (less than 10), but this region marked the predominant anatomic site for initiation of inflammation in this moderately susceptible strain. The distribution and phenotype of these cells within the retinas differed between mouse strains exhibiting different disease susceptibility. In EAU-resistant BALB/c mice, many more 33D1(+) dendritic cells were present in the normal retina but were MHC class II(low/-). Conversely, no 33D1(+) or MHC class II (+) dendriform cells could be found in the normal retinas of highly EAU-susceptible B10.RIII mice.
Conclusions: A novel population of 33D1(+) DCs was identified in normal mouse retina. The function of these cells remains to be defined, but increased numbers correlate positively with structural abnormalities in the RPE and increased resistance of the strain to EAU.
Figures


References
-
- Lohr J, Knoechel B, Nagabhushanam V, Abbas AK. T-cell tolerance and autoimmunity to systemic and tissue-restricted self-antigens. Immunol Rev. 2005;204:116–127. - PubMed
-
- Perry VH. A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation. J Neuroimmunol. 1998;90:113–121. - PubMed
-
- Camelo S, Shanley A, Voon AS, McMenamin PG. The distribution of antigen in lymphoid tissues following its injection into the anterior chamber of the rat eye. J Immunol. 2004;172:5388–5395. - PubMed
-
- Hatterer E, Davoust N, Didier-Bazes M, et al. How to drain without lymphatics? dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood. 2006;107:806–812. - PubMed
-
- Tripathi RC, Tripathi BJ. Human trabecular endothelium, corneal endothelium, keratocytes, and scleral fibroblasts in primary cell culture: a comparative study of growth characteristics, morphology, and phagocytic activity by light and scanning electron microscopy. Exp Eye Res. 1982;35:611–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

