How physicians approach prostate cancer screening before and after losing a lawsuit
- PMID: 17389535
- PMCID: PMC1838685
How physicians approach prostate cancer screening before and after losing a lawsuit
Abstract
Purpose: In 2004, a commentary by Merenstein was published in JAMA describing how he was sued for engaging a patient in shared decision making for prostate cancer screening. The article sparked considerable debate on the impact of litigation on medical care. A natural experiment (a study assessing shared decision making under way at the practice that was sued) enabled us to evaluate whether physicians changed their prostate cancer screening behavior after the lawsuit.
Methods: As part of a randomized controlled trial conducted between January 2002 and November 2004, patients and physicians completed exit questionnaires about prostate cancer screening discussions after health maintenance examinations. We compared responses before, during, and after physicians became aware of the lawsuit.
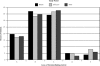
Results: A total of 432 of 497 patients completed questionnaires (180 before the practice became aware of the lawsuit, 87 as knowledge of the case diffused through the practice, and 165 after publication of Merenstein's commentary). Comparing patients' responses over the 3 time periods, there were no changes in the average locus of decision-making control, time spent discussing screening, number of screening topics discussed, knowledge scores, or decisional conflict. The frequency with which physicians reported performing prostate-specific antigen testing increased (before vs after: 84% vs 90%; P = .03), and physicians were more likely to report that they, rather than the patients, had made the screening decision (before vs after: 3.3% vs 11.1%; P = .003).
Conclusions: The physicians in closest proximity to this well-known legal case continued to engage patients in shared decision making and to let patients decide whether to be screened. Prostate-specific antigen testing increased during this period.
Figures

References
-
- Merenstein D. A piece of my mind. Winners and losers. JAMA. 2004;291(1):15–16. - PubMed
-
- Fleming M. Evidence-based medicine on trial. JAMA. 2004;291(14): 1697–1698; author reply 1698. - PubMed
-
- Hall MA, Green MD, Hartz A. Evidence-based medicine on trial. JAMA. 2004;291(14):1697; author reply 1698. - PubMed
-
- Watts C. Evidence-based medicine on trial. JAMA. 2004;291(14): 1697; author reply 1698. - PubMed
-
- Morse LJ. Evidence-based medicine on trial. JAMA. 2004;291(14): 1697; author reply 1698. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
