Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain
- PMID: 17389593
- PMCID: PMC3088512
- DOI: 10.1074/jbc.M700827200
Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain
Abstract
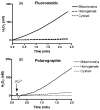
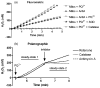
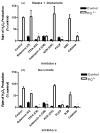
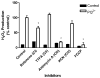
Paraquat (PQ(2+)) is a prototypic toxin known to exert injurious effects through oxidative stress and bears a structural similarity to the Parkinson disease toxicant, 1-methyl-4-pheynlpyridinium. The cellular sources of PQ(2+)-induced reactive oxygen species (ROS) production, specifically in neuronal tissue, remain to be identified. The goal of this study was to determine the involvement of brain mitochondria in PQ(2+)-induced ROS production. Highly purified rat brain mitochondria were obtained using a Percoll density gradient method. PQ(2+)-induced hydrogen peroxide (H(2)O(2)) production was measured by fluorometric and polarographic methods. The production of H(2)O(2) was evaluated in the presence of inhibitors and modulators of the mitochondrial respiratory chain. The results presented here suggest that in the rat brain, (a) mitochondria are a principal cellular site of PQ(2+)-induced H(2)O(2) production, (b) PQ(2+)-induced H(2)O(2) production requires the presence of respiratory substrates, (c) complex III of the electron transport chain is centrally involved in H(2)O(2) production by PQ(2+), and (d) the mechanism by which PQ(2+) generates H(2)O(2) depends on the mitochondrial inner transmembrane potential. These observations were further confirmed by measuring PQ(2+)-induced H(2)O(2) production in primary neuronal cells derived from the midbrain. These findings shed light on the mechanism through which mitochondria may contribute to ROS production by other environmental and endogenous redox cycling agents implicated in Parkinson's disease.
Figures
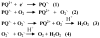
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

