Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice
- PMID: 17389906
- PMCID: PMC3392959
- DOI: 10.1038/sj.mp.4001921
Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice
Abstract
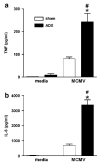
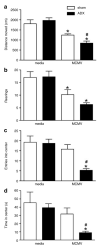
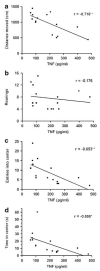
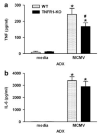
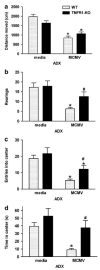
Endogenous glucocorticoids restrain proinflammatory cytokine responses to immune challenges such as viral infection. In addition, proinflammatory cytokines induce behavioral alterations including changes in locomotor/exploratory activity. Accordingly, we examined proinflammatory cytokines and open-field behavior in virally infected mice rendered glucocorticoid deficient by adrenalectomy (ADX). Mice were infected with murine cytomegalovirus (MCMV), and open-field behavior (36 h post-infection) and plasma concentrations of tumor necrosis factor (TNF)-alpha and interleukin (IL)-6 (42 h post-infection) were assessed. Compared to sham-ADX-MCMV-infected animals, ADX-MCMV-infected mice exhibited significant reductions in total distance moved, number of center entries, and time spent in center. These behavioral alterations were accompanied by significantly higher plasma concentrations of TNF-alpha and IL-6, both of which were correlated with degree of behavioral change. To examine the role of TNF-alpha in these behavioral alterations, open-field behavior was compared in wild-type (WT) and TNF-R1-knockout (KO), ADX-MCMV-infected mice. TNF-R1-KO mice exhibited significantly attenuated decreases in number of rearings, number of center entries and time spent in center, but not distance moved, which correlated with plasma IL-6. Given the potential role of brain cytokines in these findings, mRNA expression of TNF-alpha, IL-1 and IL-6 was assessed in various brain regions. Although MCMV induced increases in proinflammatory cytokine mRNA throughout the brain (especially in ADX animals), no remarkable differences were found between WT and TNF-R1-KO mice. These results demonstrate that endogenous glucocorticoids restrain proinflammatory cytokine responses to viral infection and their impact on locomotor/exploratory activity. Moreover, TNF-alpha appears to mediate cytokine-induced changes in open-field behaviors, especially those believed to reflect anxiety.
Figures

Similar articles
-
Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection.J Immunol. 1999 Mar 15;162(6):3527-33. J Immunol. 1999. PMID: 10092810
-
Tumor necrosis factor-alpha-induced apoptosis in murine cytomegalovirus retinitis.Invest Ophthalmol Vis Sci. 2007 Apr;48(4):1691-700. doi: 10.1167/iovs.06-1040. Invest Ophthalmol Vis Sci. 2007. PMID: 17389501
-
Characterization of an interleukin-6- and adrenocorticotropin-dependent, immune-to-adrenal pathway during viral infection.Endocrinology. 2004 Aug;145(8):3580-9. doi: 10.1210/en.2003-1421. Epub 2004 Mar 24. Endocrinology. 2004. PMID: 15044377
-
Immunomodulation of murine cytomegalovirus-induced myocarditis in mice treated with lipopolysaccharide and tumor necrosis factor.Cell Immunol. 2001 Oct 10;213(1):52-61. doi: 10.1006/cimm.2001.1859. Cell Immunol. 2001. PMID: 11747356
-
Use of quantitative real-time PCR (qRT-PCR) to measure cytokine transcription and viral load in murine cytomegalovirus infection.J Virol Methods. 2006 Feb;131(2):122-9. doi: 10.1016/j.jviromet.2005.07.013. Epub 2005 Sep 2. J Virol Methods. 2006. PMID: 16140399
Cited by
-
Neurobiological Mechanisms of Electroconvulsive Therapy: Molecular Perspectives of Brain Stimulation.Int J Mol Sci. 2025 Jun 19;26(12):5905. doi: 10.3390/ijms26125905. Int J Mol Sci. 2025. PMID: 40565369 Free PMC article. Review.
-
Similar therapeutic efficacy between a single administration of gene therapy and multiple administrations of recombinant enzyme in a mouse model of lysosomal storage disease.Hum Gene Ther. 2014 Jul;25(7):609-18. doi: 10.1089/hum.2013.213. Epub 2014 Apr 11. Hum Gene Ther. 2014. PMID: 24725025 Free PMC article.
-
A computational account of joint SSRI and anti-inflammatory treatment.Front Immunol. 2025 Apr 25;16:1472732. doi: 10.3389/fimmu.2025.1472732. eCollection 2025. Front Immunol. 2025. PMID: 40352929 Free PMC article.
-
Stress-related suppression of peripheral cytokines predicts future relapse in alcohol-dependent individuals with and without subclinical depression.Addict Biol. 2020 Nov;25(6):e12832. doi: 10.1111/adb.12832. Epub 2019 Nov 17. Addict Biol. 2020. PMID: 31736187 Free PMC article.
-
Immune system inflammation in cocaine dependent individuals: implications for medications development.Hum Psychopharmacol. 2012 Mar;27(2):156-66. doi: 10.1002/hup.1251. Hum Psychopharmacol. 2012. PMID: 22389080 Free PMC article. Clinical Trial.
References
-
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. - PubMed
-
- Silverman MN, Miller AH, Biron CA, Pearce BD. Characterization of an interleukin-6- and adrenocorticotropin-dependent, immune-to-adrenal pathway during viral infection. Endocrinology. 2004;145:3580–3589. - PubMed
-
- Glezer I, Rivest S. Glucocorticoids: protectors of the brain during innate immune responses. Neuroscientist. 2004;10:538–552. - PubMed
-
- Miller AH, Pearce BD, Ruzek MC, Biron CA. Interactions between the hypothalamic-pituitary-adrenal axis and immune system during viral infection: pathways for environmental effects on disease expression. In: McEwen BS, editor. Handbook of Physiology. Oxford University Press; New York: 2001. pp. 425–450.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

