Cohesin is dispensable for centromere cohesion in human cells
- PMID: 17389909
- PMCID: PMC1820851
- DOI: 10.1371/journal.pone.0000318
Cohesin is dispensable for centromere cohesion in human cells
Abstract
Background: Proper regulation of the cohesion at the centromeres of human chromosomes is essential for accurate genome transmission. Exactly how cohesion is maintained and is then dissolved in anaphase is not understood.
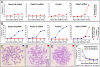
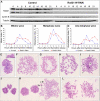
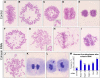
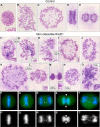
Principal findings: We have investigated the role of the cohesin complex at centromeres in human cells both by depleting cohesin subunits using RNA interference and also by expressing a non-cleavable version of the Rad21 cohesin protein. Rad21 depletion results in aberrant anaphase, during which the sister chromatids separate and segregate in an asynchronous fashion. However, centromere cohesion was maintained before anaphase in Rad21-depleted cells, and the primary constrictions at centromeres were indistinguishable from those in control cells. Expression of non-cleavable Rad21 (NC-Rad21), in which the sites normally cleaved by separase are mutated, resulted in delayed sister chromatid resolution in prophase and prometaphase, and a blockage of chromosome arm separation in anaphase, but did not impede centromere separation.
Conclusions: These data indicate that cohesin complexes are dispensable for sister cohesion in early mitosis, yet play an important part in the fidelity of sister separation and segregation during anaphase. Cleavage at the separase-sensitive sites of Rad21 is important for arm separation, but not for centromere separation.
Conflict of interest statement
Figures

Similar articles
-
Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells.PLoS Biol. 2005 Mar;3(3):e86. doi: 10.1371/journal.pbio.0030086. Epub 2005 Mar 1. PLoS Biol. 2005. PMID: 15737064 Free PMC article.
-
The complete removal of cohesin from chromosome arms depends on separase.J Cell Sci. 2007 Dec 1;120(Pt 23):4188-96. doi: 10.1242/jcs.011528. Epub 2007 Nov 14. J Cell Sci. 2007. PMID: 18003702
-
Calpain-1 cleaves Rad21 to promote sister chromatid separation.Mol Cell Biol. 2011 Nov;31(21):4335-47. doi: 10.1128/MCB.06075-11. Epub 2011 Aug 29. Mol Cell Biol. 2011. PMID: 21876002 Free PMC article.
-
Shugoshin: guardian spirit at the centromere.Curr Opin Cell Biol. 2005 Dec;17(6):590-5. doi: 10.1016/j.ceb.2005.10.003. Epub 2005 Oct 17. Curr Opin Cell Biol. 2005. PMID: 16229998 Review.
-
Shugoshin, a guardian for sister chromatid segregation.Exp Cell Res. 2005 Oct 15;310(1):1-9. doi: 10.1016/j.yexcr.2005.07.018. Exp Cell Res. 2005. PMID: 16112668 Review.
Cited by
-
Investigating the Interplay between Sister Chromatid Cohesion and Homolog Pairing in Drosophila Nuclei.PLoS Genet. 2016 Aug 19;12(8):e1006169. doi: 10.1371/journal.pgen.1006169. eCollection 2016 Aug. PLoS Genet. 2016. PMID: 27541002 Free PMC article.
-
Cohesin promotes the repair of ionizing radiation-induced DNA double-strand breaks in replicated chromatin.Nucleic Acids Res. 2010 Jan;38(2):477-87. doi: 10.1093/nar/gkp976. Epub 2009 Nov 11. Nucleic Acids Res. 2010. PMID: 19906707 Free PMC article.
-
Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes.Genes Dev. 2010 Nov 15;24(22):2505-16. doi: 10.1101/gad.605910. Epub 2010 Oct 22. Genes Dev. 2010. PMID: 20971813 Free PMC article.
-
Perturbing cohesin dynamics drives MRE11 nuclease-dependent replication fork slowing.Nucleic Acids Res. 2019 Feb 20;47(3):1294-1310. doi: 10.1093/nar/gky519. Nucleic Acids Res. 2019. PMID: 29917110 Free PMC article.
-
Role of helicase-like transcription factor (hltf) in the G2/m transition and apoptosis in brain.PLoS One. 2013 Jun 24;8(6):e66799. doi: 10.1371/journal.pone.0066799. Print 2013. PLoS One. 2013. PMID: 23826137 Free PMC article.
References
-
- Uhlmann F, Nasmyth K. Cohesion between sister chromatids must be established during DNA replication. Curr Biol. 1998;8:1095–1101. - PubMed

