Serial killing of tumor cells by human natural killer cells--enhancement by therapeutic antibodies
- PMID: 17389917
- PMCID: PMC1828617
- DOI: 10.1371/journal.pone.0000326
Serial killing of tumor cells by human natural killer cells--enhancement by therapeutic antibodies
Abstract
Background: Natural killer cells are an important component of the innate immune system. Anti-cancer therapies utilizing monoclonal antibodies also rely on the cytotoxicity of NK cells for their effectiveness. Here, we study the dynamics of NK cell cytotoxicity.
Methodology/principal findings: We observe that IL-2 activated human NK cells can serially hit multiple targets. Using functional assays, we demonstrate that on an average, a single IL-2 activated NK cell can kill four target cells. Data using live video microscopy suggest that an individual NK cell can make serial contacts with multiple targets and majority of contacts lead to lysis of target cells. Serial killing is associated with a loss of Perforin and Granzyme B content. A large majority of NK cells survive serial killing, and IL-2 can replenish their granular stock and restore the diminished cytotoxicity of 'exhausted' NK cells. IL-2 and IL-15 are equally effective in enhancing the killing frequency of resting NK cells. Significantly, Rituximab, a therapeutic monoclonal antibody increases the killing frequency of both resting and IL-2 activated NK cells.
Conclusion/significance: Our data suggest that NK cell-based therapies for overcoming tumors rely on their serial killing ability. Therefore, strategies augmenting the killing ability of NK cells can boost the immune system and enhance the effectiveness of monoclonal antibody-based therapies.
Conflict of interest statement
Figures
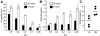


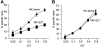

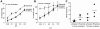
References
-
- Perelson AS, Macken CA, Grimm EA, Roos LS, Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. VIII. Kinetics of lysis of target cells bound by more than one cytotoxic T lymphocyte. J Immunol. 1984;132:2190–2198. - PubMed
-
- Martz E. Multiple target cell killing by the cytolytic T lymphocyte and the mechanism of cytotoxicity. Transplantation. 1976;21:5–11. - PubMed
-
- Perelson AS, Bell GI. Delivery of lethal hits by cytotoxic T lymphocytes in multicellular conjugates occurs sequentially but at random times. J Immunol. 1982;129:2796–2801. - PubMed
-
- Thorn RM, Henney CS. Kinetic analysis of target cell destruction by effector T cells. I. Delineation of parameters related to the frequency and lytic efficiency of killer cells. J Immunol. 1976;117:2213–2219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

