Statistical reviewers improve reporting in biomedical articles: a randomized trial
- PMID: 17389922
- PMCID: PMC1824709
- DOI: 10.1371/journal.pone.0000332
Statistical reviewers improve reporting in biomedical articles: a randomized trial
Abstract
Background: Although peer review is widely considered to be the most credible way of selecting manuscripts and improving the quality of accepted papers in scientific journals, there is little evidence to support its use. Our aim was to estimate the effects on manuscript quality of either adding a statistical peer reviewer or suggesting the use of checklists such as CONSORT or STARD to clinical reviewers or both.
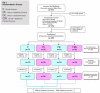
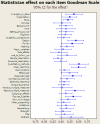
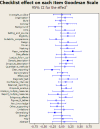
Methodology and principal findings: Interventions were defined as 1) the addition of a statistical reviewer to the clinical peer review process, and 2) suggesting reporting guidelines to reviewers; with "no statistical expert" and "no checklist" as controls. The two interventions were crossed in a 2x2 balanced factorial design including original research articles consecutively selected, between May 2004 and March 2005, by the Medicina Clinica (Barc) editorial committee. We randomized manuscripts to minimize differences in terms of baseline quality and type of study (intervention, longitudinal, cross-sectional, others). Sample-size calculations indicated that 100 papers provide an 80% power to test a 55% standardized difference. We specified the main outcome as the increment in quality of papers as measured on the Goodman Scale. Two blinded evaluators rated the quality of manuscripts at initial submission and final post peer review version. Of the 327 manuscripts submitted to the journal, 131 were accepted for further review, and 129 were randomized. Of those, 14 that were lost to follow-up showed no differences in initial quality to the followed-up papers. Hence, 115 were included in the main analysis, with 16 rejected for publication after peer review. 21 (18.3%) of the 115 included papers were interventions, 46 (40.0%) were longitudinal designs, 28 (24.3%) cross-sectional and 20 (17.4%) others. The 16 (13.9%) rejected papers had a significantly lower initial score on the overall Goodman scale than accepted papers (difference 15.0, 95% CI: 4.6-24.4). The effect of suggesting a guideline to the reviewers had no effect on change in overall quality as measured by the Goodman scale (0.9, 95% CI: -0.3-+2.1). The estimated effect of adding a statistical reviewer was 5.5 (95% CI: 4.3-6.7), showing a significant improvement in quality.
Conclusions and significance: This prospective randomized study shows the positive effect of adding a statistical reviewer to the field-expert peers in improving manuscript quality. We did not find a statistically significant positive effect by suggesting reviewers use reporting guidelines.
Conflict of interest statement
Figures


Similar articles
-
Reviewer training for improving grant and journal peer review.Cochrane Database Syst Rev. 2023 Nov 28;11(11):MR000056. doi: 10.1002/14651858.MR000056.pub2. Cochrane Database Syst Rev. 2023. PMID: 38014743 Free PMC article.
-
[Effect of statistical review on manuscript quality in Medicina Clínica (Barcelona): a randomized study].Med Clin (Barc). 2003 Nov 22;121(18):690-4. doi: 10.1016/s0025-7753(03)74064-0. Med Clin (Barc). 2003. PMID: 14651815 Clinical Trial. Spanish.
-
Peer-review and editorial process of the Ethiopian Medical Journal: ten years assessment of the status of submitted manuscripts.Ethiop Med J. 2013 Apr;51(2):95-103. Ethiop Med J. 2013. PMID: 24079153
-
What feedback do reviewers give when reviewing qualitative manuscripts? A focused mapping review and synthesis.BMC Med Res Methodol. 2020 May 18;20(1):122. doi: 10.1186/s12874-020-01005-y. BMC Med Res Methodol. 2020. PMID: 32423388 Free PMC article. Review.
-
Quality of reporting and fragility index for randomized controlled trials in the vesicoureteral reflux literature: where do we stand?J Pediatr Urol. 2019 May;15(3):204-212. doi: 10.1016/j.jpurol.2019.02.014. Epub 2019 Mar 7. J Pediatr Urol. 2019. PMID: 31060965 Review.
Cited by
-
Outcomes Definitions and Statistical Tests in Oncology Studies: A Systematic Review of the Reporting Consistency.PLoS One. 2016 Oct 7;11(10):e0164275. doi: 10.1371/journal.pone.0164275. eCollection 2016. PLoS One. 2016. PMID: 27716793 Free PMC article.
-
Enhancing biostatistics education for medical students in Poland: factors influencing perception and educational recommendations.BMC Med Educ. 2024 Apr 22;24(1):428. doi: 10.1186/s12909-024-05389-z. BMC Med Educ. 2024. PMID: 38649993 Free PMC article.
-
There is no reliable evidence that providing authors with customized article templates including items from reporting guidelines improves completeness of reporting: the GoodReports randomized trial (GRReaT).BMC Med Res Methodol. 2025 Mar 14;25(1):71. doi: 10.1186/s12874-025-02518-0. BMC Med Res Methodol. 2025. PMID: 40087548 Free PMC article. Clinical Trial.
-
Non-publication of large randomized clinical trials: cross sectional analysis.BMJ. 2013 Oct 29;347:f6104. doi: 10.1136/bmj.f6104. BMJ. 2013. PMID: 24169943 Free PMC article. Review.
-
Impact of STROBE statement publication on quality of observational study reporting: interrupted time series versus before-after analysis.PLoS One. 2013 Aug 26;8(8):e64733. doi: 10.1371/journal.pone.0064733. eCollection 2013. PLoS One. 2013. PMID: 23990867 Free PMC article.
References
-
- Editorial Peering into the review process. Nat Struct Biol. 2000;7:1075–1076. - PubMed
-
- Scarpa T. Peer Review at NIH. Science. 2006;311:41. - PubMed
-
- Rennie D. Editorial peer review: let us put it on trial. Controlled Clinical Trials. 1992;13:443–445. - PubMed
-
- Editorial Bad peer reviewers. Nature. 2001;413:93. - PubMed
-
- Lock S. Does Editorial Peer Review Work? Ann Intern Med. 1994;121:60–61. - PubMed

