Dose escalating safety study of CNS 5161 HCl, a new neuronal glutamate receptor antagonist (NMDA) for the treatment of neuropathic pain
- PMID: 17391323
- PMCID: PMC2000615
- DOI: 10.1111/j.1365-2125.2007.02880.x
Dose escalating safety study of CNS 5161 HCl, a new neuronal glutamate receptor antagonist (NMDA) for the treatment of neuropathic pain
Abstract
Aims: The purpose of the current study was to establish the safety and maximal tolerated dose of CNS 5161 HCl.
Methods: Forty patients with chronic neuropathic pain (23 male, 17 female) were treated with escalating dosages of CNS 5161. All adverse events to study drug, blood pressure, heart rate, ECG, drug level and clinical laboratory values were monitored. Actual pain was measured on a 100-mm visual analogue scale (VAS) and ordinal verbal pain scores.
Results: The most commonly occurring nervous system disorder was headache, which was found more often during placebo than during CNS 5161 HCl treatment. Visual disturbances were experienced by 16.7% of patients receiving 250 microg and by 33.3% receiving 500 microg CNS 5161 HCl, but not during placebo treatment. An increase in blood pressure was observed in 8.3% of patients receiving 250 microg and in 50% of patients receiving 500 microg CNS 5161 HCl, compared with 15.4% during placebo treatment. The study was abandoned after two patients entered the 750 microg cohort due to a sustained systolic blood pressure response. Although this study was underpowered for the confirmation of efficacy, some indications of greater pain relief after 500 microg CNS 5161 compared with placebo could be observed (change in VAS between baseline and 12 h 10 +/- 22 mm vs. 2 +/- 19 mm; P = 0.11).
Conclusions: CNS 5161 HCl was reasonably well tolerated up to 500 microg. The most common adverse events were hypertension, headache and mild visual disorders.
Figures
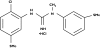
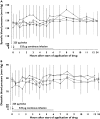
 , 125 µg;
, 125 µg;  , 250 µg;
, 250 µg;  , 500 µg CNS 5161 HCl; □, placebo)
, 500 µg CNS 5161 HCl; □, placebo)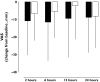
References
-
- Woolf CJ. Dissecting out mechanisms responsible for peripheral neuropathic pain: implications for diagnosis and therapy. Life Sci. 2004;74:2605–10. - PubMed
-
- Singleton JR. Evaluation and treatment of painful peripheral polyneuropathy. Semin Neurol. 2005;25:185–95. - PubMed
-
- Pud D, Eisenberg E, Spitzer A, Adler R, Fried G, Yarnitsky D. The NMDA receptor antagonist amantadine reduces surgical neuropathic pain in cancer patients: a double blind, randomized, placebo controlled trial. Pain. 1998;75:349–54. - PubMed
-
- Stannard CF, Porter GE. Ketamine hydrochloride in the treatment of phantom limb pain. Pain. 1993;54:227–30. - PubMed
-
- Backonja M, Arndt G, Gombar KA, Check B, Zimmermann M. Response of chronic neuropathic pain syndromes to ketamine: a preliminary study. Pain. 1994;56:51–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

