Autoantibodies to vimentin cause accelerated rejection of cardiac allografts
- PMID: 17392180
- PMCID: PMC1829474
- DOI: 10.2353/ajpath.2007.060728
Autoantibodies to vimentin cause accelerated rejection of cardiac allografts
Abstract
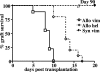
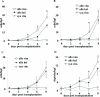
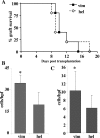
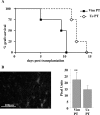
Autoimmune responses to vimentin occur after solid organ transplantation, but their pathogenic effects are unclear. The aim of these studies was to investigate the effects of vimentin preimmunization on allogeneic and isografted hearts in a murine transplant model. Immunization of C57BL/6 mice with murine vimentin in complete Freund's adjuvant resulted in anti-vimentin antibodies and vimentin-reactive Th-1 cells. Transplantation of 129/sv hearts into vimentin-immunized C57BL/6 recipients resulted in accelerated rejection (8.4 +/- 1.5 days; n = 18), compared with hen egg lysozyme-immunized C57BL/6 (13.3 +/- 2.2 days; n = 10; P < 0.0001, log-rank test). In contrast, isografts continued to beat beyond 90 days. Immunohistochemical analysis of allografts from vimentin/complete Freund's adjuvant mice demonstrated increased numbers of T cells and enhanced microvascular deposition of C3d, CD41, and P-selectin compared with controls. Antibodies were necessary for accelerated rejection, shown by the fact that vimentin-immunized B-cell-deficient IgH6 mice did not show accelerated rejection of 129/sv allografts, but rejection was restored by adoptive transfer of serum containing anti-vimentin antibodies. Eluates from donor hearts placed in vimentin/complete Freund's adjuvant recipients contained anti-vimentin antibodies, shown by Western blotting. Confocal imaging of rejected hearts de-monstrated presence of vimentin and C3d on apoptosed leukocytes, endothelial cells, and platelet/leukocyte conjugates. These results demonstrate that autoantibodies to vimentin, in conjunction with the alloimmune response, have a pathogenic role in allograft rejection.
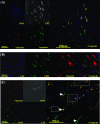
Figures




References
-
- Taylor DO, Edwards LB, Boucek MM, Trulock EP, Keck BM, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report–2004. J Heart Lung Transplant. 2004;23:796–803. - PubMed
-
- Chapman JR, O’Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005;16:3015–3026. - PubMed
-
- Hornick P, Lechler R. Direct and indirect pathways of alloantigen recognition: relevance to acute and chronic allograft rejection. Nephrol Dial Transplant. 1997;12:1806–1810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

